PINK1 and Parkin complementarily protect dopaminergic neurons in vertebrates
- PMID: 23449626
- PMCID: PMC10259650
- DOI: 10.1093/hmg/ddt095
PINK1 and Parkin complementarily protect dopaminergic neurons in vertebrates
Abstract
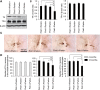
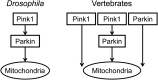
Parkinson's disease (PD) is a common neurodegenerative disorder characterized by selective dopaminergic cell loss in the substantia nigra, but its pathogenesis remains unclear. The recessively inherited familial PD genes PARK2 and PARK6 have been attributed to mutations in the Parkin and PTEN-induced kinase 1 (PINK1) genes, respectively. Recent reports suggest that PINK1 works upstream of Parkin in the same pathway to regulate mitochondrial dynamics and/or conduct autophagic clearance of damaged mitochondria. This phenomenon is preserved from Drosophila to human cell lines but has not been demonstrated in a vertebrate animal model in vivo. Here, we developed a medaka fish (Oryzias latipes) model that is deficient in Pink1 and Parkin. We found that despite the lack of a conspicuous phenotype in single mutants for Pink1 or Parkin, medaka that are deficient in both genes developed phenotypes similar to that of human PD: late-onset locomotor dysfunction, a decrease in dopamine levels and a selective degeneration of dopaminergic neurons. Further analysis also revealed defects in mitochondrial enzymatic activity as well as cell death. Consistently, PINK1 and Parkin double-deficient MEF showed a further decrease in mitochondrial membrane potential and mitochondrial complex I activity as well as apoptosis compared with single-deficient MEF. Interestingly, these mitochondrial abnormalities in Parkin-deficient MEF were compensated by exogenous PINK1, but not by disease-related mutants. These results suggest that PINK1 and Parkin work in a complementary way to protect dopaminergic neurons by maintaining mitochondrial function in vertebrates.
Figures





Similar articles
-
Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin.Nature. 2006 Jun 29;441(7097):1162-6. doi: 10.1038/nature04779. Epub 2006 May 3. Nature. 2006. PMID: 16672981
-
Loss of PINK1 in medaka fish (Oryzias latipes) causes late-onset decrease in spontaneous movement.Neurosci Res. 2010 Feb;66(2):151-61. doi: 10.1016/j.neures.2009.10.010. Epub 2009 Nov 4. Neurosci Res. 2010. PMID: 19895857
-
PINK1-Parkin signaling in Parkinson's disease: Lessons from Drosophila.Neurosci Res. 2020 Oct;159:40-46. doi: 10.1016/j.neures.2020.01.016. Epub 2020 Feb 6. Neurosci Res. 2020. PMID: 32035987 Review.
-
The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons.Hum Mol Genet. 2011 Aug 15;20(16):3227-40. doi: 10.1093/hmg/ddr235. Epub 2011 May 25. Hum Mol Genet. 2011. PMID: 21613270 Free PMC article.
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
Cited by
-
TMEM175 deficiency impairs lysosomal and mitochondrial function and increases α-synuclein aggregation.Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2389-2394. doi: 10.1073/pnas.1616332114. Epub 2017 Feb 13. Proc Natl Acad Sci U S A. 2017. PMID: 28193887 Free PMC article.
-
Targeted Treatment Strategies for Mitochondria Dysfunction: Correlation with Neurological Disorders.Curr Drug Targets. 2024;25(10):683-699. doi: 10.2174/0113894501303824240604103732. Curr Drug Targets. 2024. PMID: 38910425 Review.
-
Potentiation of neurotoxicity in double-mutant mice with Pink1 ablation and A53T-SNCA overexpression.Hum Mol Genet. 2015 Feb 15;24(4):1061-76. doi: 10.1093/hmg/ddu520. Epub 2014 Oct 8. Hum Mol Genet. 2015. PMID: 25296918 Free PMC article.
-
Inducing mitophagy in diabetic platelets protects against severe oxidative stress.EMBO Mol Med. 2016 Jul 1;8(7):779-95. doi: 10.15252/emmm.201506046. Print 2016 Jul. EMBO Mol Med. 2016. PMID: 27221050 Free PMC article.
-
Viable neuronopathic Gaucher disease model in Medaka (Oryzias latipes) displays axonal accumulation of alpha-synuclein.PLoS Genet. 2015 Apr 2;11(4):e1005065. doi: 10.1371/journal.pgen.1005065. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25835295 Free PMC article.
References
-
- Kitada T. Asakawa S. Hattori N. Matsumine H. Yamamura Y. Minoshima S. Yokochi M. Mizuno Y. Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. - PubMed
-
- Valente E.M. Abou-Sleiman P.M. Caputo V. Muqit M.M. Harvey K. Gispert S. Ali Z. Del Turco D. Bentivoglio A.R. Healy D.G. et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. - PubMed
-
- Clark I.E. Dodson M.W. Jiang C. Cao J.H. Huh J.R. Seol J.H. Yoo S.J. Hay B.A. Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. - PubMed
-
- Park J. Lee S.B. Lee S. Kim Y. Song S. Kim S. Bae E. Kim J. Shong M. Kim J.M. et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

