MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program
- PMID: 23449635
- PMCID: PMC3709650
- DOI: 10.1182/blood-2012-10-460063
MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program
Abstract
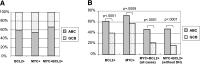
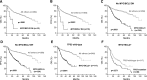
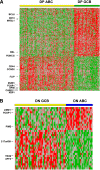
Diffuse large B-cell lymphoma (DLBCL) is stratified into prognostically favorable germinal center B-cell (GCB)-like and unfavorable activated B-cell (ABC)-like subtypes based on gene expression signatures. In this study, we analyzed 893 de novo DLBCL patients treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). We show that MYC/BCL2 protein coexpression occurred significantly more commonly in the ABC subtype. Patients with the ABC or GCB subtype of DLBCL had similar prognoses with MYC/BCL2 coexpression and without MYC/BCL2 coexpression. Consistent with the notion that the prognostic difference between the 2 subtypes is attributable to MYC/BCL2 coexpression, there is no difference in gene expression signatures between the 2 subtypes in the absence of MYC/BCL2 coexpression. DLBCL with MYC/BCL2 coexpression demonstrated a signature of marked downregulation of genes encoding extracellular matrix proteins, those involving matrix deposition/remodeling and cell adhesion, and upregulation of proliferation-associated genes. We conclude that MYC/BCL2 coexpression in DLBCL is associated with an aggressive clinical course, is more common in the ABC subtype, and contributes to the overall inferior prognosis of patients with ABC-DLBCL. In conclusion, the data suggest that MYC/BCL2 coexpression, rather than cell-of-origin classification, is a better predictor of prognosis in patients with DLBCL treated with R-CHOP.
Figures




References
-
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. - PubMed
-
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. - PubMed
-
- Muris JJ, Meijer CJ, Vos W, et al. Immunohistochemical profiling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. J Pathol. 2006;208(5):714–723. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

