Toll-like receptor-2/6 and Toll-like receptor-9 agonists suppress viral replication but not airway hyperreactivity in guinea pigs
- PMID: 23449736
- PMCID: PMC3727870
- DOI: 10.1165/rcmb.2012-0498OC
Toll-like receptor-2/6 and Toll-like receptor-9 agonists suppress viral replication but not airway hyperreactivity in guinea pigs
Abstract
Respiratory virus infections cause airway hyperreactivity (AHR). Preventative strategies for virus-induced AHR remain limited. Toll-like receptors (TLRs) have been suggested as a therapeutic target because of their central role in triggering antiviral immune responses. Previous studies showed that concurrent treatment with TLR2/6 and TLR9 agonists reduced lethality and the microbial burden in murine models of bacterial and viral pneumonia. This study investigated the effects of TLR2/6 and TLR9 agonist pretreatment on parainfluenza virus pneumonia and virus-induced AHR in guinea pigs in vivo. Synthetic TLR2/6 lipopeptide agonist Pam₂CSK₄ and Class C oligodeoxynucleotide TLR9 agonist ODN2395, administered in combination 24 hours before virus infection, significantly reduced viral replication in the lung. Despite a fivefold reduction in viral titers, concurrent TLR2/6 and TLR9 agonist pretreatment did not prevent virus-induced AHR or virus-induced inhibitory M2 muscarinic receptor dysfunction. Interestingly, the TLR agonists independently caused non-M2-dependent AHR. These data confirm the therapeutic antiviral potential of TLR agonists, while suggesting that virus inhibition may be insufficient to prevent virus-induced airway pathophysiology. Furthermore, TLR agonists independently cause AHR, albeit through a distinctly different mechanism from that of parainfluenza virus.
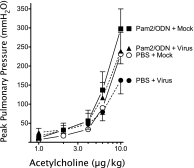
Figures





References
-
- Atmar RL, Guy E, Guntupalli KK, Zimmerman JL, Bandi VD, Baxter BD, Greenberg SB. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–2459. - PubMed
-
- Kherad O, Bridevaux PO, Kaiser L, Janssens JP, Rutschmann O. Viral infection in acute exacerbation of COPD. Rev Med Suisse. 2011;7:2222–2226. - PubMed
-
- Aquilina AT, Hall WJ, Douglas RG, Jr, Utell MJ. Airway reactivity in subjects with viral upper respiratory tract infections: the effects of exercise and cold air. Am Rev Respir Dis. 1980;122:3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T-32 HL-83808/HL/NHLBI NIH HHS/United States
- R01 HL055543/HL/NHLBI NIH HHS/United States
- DP2 HL123229/HL/NHLBI NIH HHS/United States
- R01 ES014601/ES/NIEHS NIH HHS/United States
- HL115903/HL/NHLBI NIH HHS/United States
- R44 HL115903/HL/NHLBI NIH HHS/United States
- ES014601/ES/NIEHS NIH HHS/United States
- HL54659/HL/NHLBI NIH HHS/United States
- R43 HL115903/HL/NHLBI NIH HHS/United States
- R01 HL117976/HL/NHLBI NIH HHS/United States
- R01 HL054659/HL/NHLBI NIH HHS/United States
- R01 ES017592/ES/NIEHS NIH HHS/United States
- HL071795/HL/NHLBI NIH HHS/United States
- R01 HL113023/HL/NHLBI NIH HHS/United States
- T32 HL083808/HL/NHLBI NIH HHS/United States
- HL55543/HL/NHLBI NIH HHS/United States
- R01 HL071795/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

