Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury
- PMID: 23449739
- PMCID: PMC3727889
- DOI: 10.1165/rcmb.2012-0411TR
Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury
Abstract
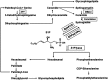
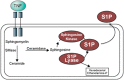
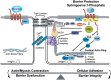
Acute lung injury (ALI) attributable to sepsis or mechanical ventilation and subacute lung injury because of ionizing radiation (RILI) share profound increases in vascular permeability as a key element and a common pathway driving increased morbidity and mortality. Unfortunately, despite advances in the understanding of lung pathophysiology, specific therapies do not yet exist for the treatment of ALI or RILI, or for the alleviation of unremitting pulmonary leakage, which serves as a defining feature of the illness. A critical need exists for new mechanistic insights that can lead to novel strategies, biomarkers, and therapies to reduce lung injury. Sphingosine 1-phosphate (S1P) is a naturally occurring bioactive sphingolipid that acts extracellularly via its G protein-coupled S1P1-5 as well as intracellularly on various targets. S1P-mediated cellular responses are regulated by the synthesis of S1P, catalyzed by sphingosine kinases 1 and 2, and by the degradation of S1P mediated by lipid phosphate phosphatases, S1P phosphatases, and S1P lyase. We and others have demonstrated that S1P is a potent angiogenic factor that enhances lung endothelial cell integrity and an inhibitor of vascular permeability and alveolar flooding in preclinical animal models of ALI. In addition to S1P, S1P analogues such as 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol (FTY720), FTY720 phosphate, and FTY720 phosphonates offer therapeutic potential in murine models of lung injury. This translational review summarizes the roles of S1P, S1P analogues, S1P-metabolizing enzymes, and S1P receptors in the pathophysiology of lung injury, with particular emphasis on the development of potential novel biomarkers and S1P-based therapies for ALI and RILI.
Figures




Similar articles
-
Epigenetic regulation of pro-inflammatory cytokine secretion by sphingosine 1-phosphate (S1P) in acute lung injury: Role of S1P lyase.Adv Biol Regul. 2017 Jan;63:156-166. doi: 10.1016/j.jbior.2016.09.007. Epub 2016 Sep 29. Adv Biol Regul. 2017. PMID: 27720306 Free PMC article. Review.
-
Targeting sphingosine-1-phosphate in hematologic malignancies.Anticancer Agents Med Chem. 2011 Nov;11(9):794-8. doi: 10.2174/187152011797655122. Anticancer Agents Med Chem. 2011. PMID: 21707492 Free PMC article. Review.
-
Functional characterization of sphingosine 1-phosphate receptor agonist in human endothelial cells.Prostaglandins Other Lipid Mediat. 2004 Jan;73(1-2):29-45. doi: 10.1016/j.prostaglandins.2003.11.003. Prostaglandins Other Lipid Mediat. 2004. PMID: 15165029
-
Targeting sphingosine-1-phosphate signaling in lung diseases.Pharmacol Ther. 2016 Dec;168:143-157. doi: 10.1016/j.pharmthera.2016.09.008. Epub 2016 Sep 13. Pharmacol Ther. 2016. PMID: 27621206 Free PMC article. Review.
-
FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury.Crit Care Med. 2014 Mar;42(3):e189-99. doi: 10.1097/CCM.0000000000000097. Crit Care Med. 2014. PMID: 24335440 Free PMC article.
Cited by
-
Genomic and Genetic Approaches to Deciphering Acute Respiratory Distress Syndrome Risk and Mortality.Antioxid Redox Signal. 2019 Nov 10;31(14):1027-1052. doi: 10.1089/ars.2018.7701. Epub 2019 Jun 18. Antioxid Redox Signal. 2019. PMID: 31016989 Free PMC article. Review.
-
Mind the Gap between the Endothelium and E3 Ubiquitin Ligase: TRIM21 Is a Viable Therapeutic Target in Sepsis-induced Endothelial Dysfunction.Am J Respir Cell Mol Biol. 2019 Dec;61(6):676-677. doi: 10.1165/rcmb.2019-0161ED. Am J Respir Cell Mol Biol. 2019. PMID: 31199667 Free PMC article. No abstract available.
-
Linarin prevents LPS‑induced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NF‑κB pathways.Int J Mol Med. 2018 Sep;42(3):1460-1472. doi: 10.3892/ijmm.2018.3710. Epub 2018 May 30. Int J Mol Med. 2018. PMID: 29845284 Free PMC article.
-
Sphingolipids in Ventilator Induced Lung Injury: Role of Sphingosine-1-Phosphate Lyase.Int J Mol Sci. 2018 Jan 1;19(1):114. doi: 10.3390/ijms19010114. Int J Mol Sci. 2018. PMID: 29301259 Free PMC article.
-
Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury.Thorax. 2019 Jun;74(6):579-591. doi: 10.1136/thoraxjnl-2018-212378. Epub 2019 Feb 5. Thorax. 2019. PMID: 30723184 Free PMC article.
References
-
- Goss CH, Brower RG, Hudson LD, Rubenfeld GD. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. - PubMed
-
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. - PubMed
-
- Merrill AH, Schmelz E-M, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley KA, Wang E. Sphingolipids—the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol. 1997;142:208–225. - PubMed
-
- Uhlig S. Gulbins: sphingolipids in the lungs. Am J Respir Crit Care Med. 2008;178:1100–1114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

