Immunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trials
- PMID: 23449768
- PMCID: PMC3641615
- DOI: 10.2215/CJN.07570712
Immunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trials
Abstract
Background and objectives: The efficacy and safety of immunosuppression for idiopathic membranous nephropathy (IMN) with nephrotic syndrome are still controversial. A systematic review and meta-analysis of randomized controlled trials (RCTs) was performed.
Design, setting, participants, & measurements: The Cochrane Library, PUBMED, EMBASE, Chinese Database, and Clinical Trial Registries (June 2012) were searched to identify RCTs investigating the effect of immunosuppression on adults with IMN and nephrotic syndrome.
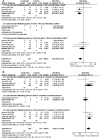
Results: This review was an update (36 RCTs, 1762 participants) of the 2004 version (18 RCTs, 1025 participants). Immunosuppression significantly reduced all-cause mortality or ESRD (15 RCTs, 791 participants; risk ratio, 0.58 [95% confidence interval, 0.36-0.95]; P=0.03). However, the result was not consistent when prespecified subgroup analyses were undertaken. Immunosuppression increased complete or partial remission (CR + PR) (16 RCTs, 864 participants; 1.31 [1.01-1.70]; P=0.04) but resulted in more withdrawals or hospitalizations (16 RCTs, 880 participants; 5.35 [2.19-13.02]; P=0.002). Corticosteroids combined with alkylating agents significantly reduced all-cause mortality or ESRD (8 RCTs, 448 participants; 0.44 [0.26-0.75]; P=0.002) and increased CR + PR (7 RCTs, 422 participants; 1.46 [1.13-1.89]; P=0.004) but led to more adverse events (4 RCTs, 303 participants; 4.20 [1.15-15.32]; P=0.03). Cyclophosphamide was safer than chlorambucil (3 RCTs, 147 participants; 0.48 [0.26-0.90]; P=0.02). Cyclosporine and mycophenolate mofetil failed to show superiority over alkylating agents. Tacrolimus and adrenocorticotropic hormone significantly reduced proteinuria.
Conclusions: Alkylating agents plus corticosteroids had long-term and short-term benefits for adult IMN, but resulted in more withdrawals or hospitalizations.
Figures


References
-
- Hofstra JM, Wetzels JF: Management of patients with membranous nephropathy. Nephrol Dial Transplant 27: 6–9, 2012 - PubMed
-
- Ponticelli C, Passerini P: Management of idiopathic membranous nephropathy. Expert Opin Pharmacother 11: 2163–2175, 2010 - PubMed
-
- Hofstra JM, Wetzels JF: Alkylating agents in membranous nephropathy: Efficacy proven beyond doubt. Nephrol Dial Transplant 25: 1760–1766, 2010 - PubMed
-
- Couchoud C, Laville M, Boissel JP: Treatment of membranous nephropathy: A meta-analysis. Nephrol Dial Transplant 9: 469–470, 1994 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

