A systems biology examination of the human female genital tract shows compartmentalization of immune factor expression
- PMID: 23449785
- PMCID: PMC3624310
- DOI: 10.1128/JVI.03347-12
A systems biology examination of the human female genital tract shows compartmentalization of immune factor expression
Abstract
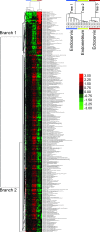
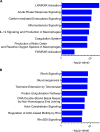
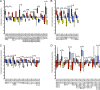
Many mucosal factors in the female genital tract (FGT) have been associated with HIV susceptibility, but little is known about their anatomical distribution in the FGT compartments. This study comprehensively characterized global immune factor expression in different tissue sites of the lower and upper FGT by using a systems biology approach. Tissue sections from the ectocervix, endocervix, and endometrium from seven women who underwent hysterectomy were analyzed by a combination of quantitative mass spectrometry and immunohistochemical staining. Of the >1,000 proteins identified, 281 were found to be differentially abundant in different tissue sites. Hierarchical clustering identified four major functional pathways distinguishing compartments, including innate immune pathways (acute-phase response, LXR/RXR) and development (RhoA signaling, gluconeogenesis), which were enriched in the ectocervix/endocervix and endometrium, respectively. Immune factors important for HIV susceptibility, including antiproteases, immunoglobulins, complement components, and antimicrobial factors, were most abundant in the ectocervix/endocervix, while the endometrium had a greater abundance of certain factors that promote HIV replication. Immune factor abundance is heterogeneous throughout the FGT and shows unique immune microenvironments for HIV based on the exposure site. This may have important implications for early events in HIV transmission and site-specific susceptibility to HIV in the FGT.
Figures



References
-
- UNAIDS 2011. Global report: UNAIDS report on the global AIDS epidemic, 2010. Joint United Nations Programme on HIV/AIDS, New York, NY: http://www.unaids.org/documents/20101123_globalreport_em.pdf
-
- Valore EV, Park CH, Igreti SL, Ganz T. 2002. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 187:561–568 - PubMed
-
- Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. 2010. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One 5:e11366 doi:10.1371/journal.pone.0011366 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

