H2AX phosphorylation is important for LANA-mediated Kaposi's sarcoma-associated herpesvirus episome persistence
- PMID: 23449797
- PMCID: PMC3624323
- DOI: 10.1128/JVI.03575-12
H2AX phosphorylation is important for LANA-mediated Kaposi's sarcoma-associated herpesvirus episome persistence
Abstract
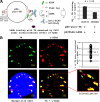
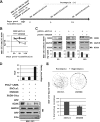
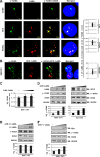
The DNA damage response (DDR) of host cells is utilized by a number of viruses to establish and propagate their genomes in the infected cells. We examined the expression of the DDR genes during Kaposi's sarcoma-associated herpesvirus (KSHV) infection of human peripheral blood mononuclear cells (PBMCs). The genes were mostly downregulated, except H2AX, which was upregulated during infection. H2AX is important for gammaherpesvirus infectivity, and its phosphorylation at serine 139 is crucial for maintenance of latency during mouse gamma-herpesvirus 68 (MHV-68) infection. We now also observed phosphorylation of H2AX at serine 139 during KSHV infection. H2AX is a histone H2A isoform shown to interact with the latency-associated nuclear antigen (LANA) encoded by KSHV. Here, we show that LANA directly interacted with H2AX through domains at both its N and C termini. The phosphorylated form of H2AX (γH2AX) was shown to colocalize with LANA. Chromatin immunoprecipitation (ChIP) assays showed that a reduction in H2AX levels resulted in reduced binding of LANA with KSHV terminal repeats (TRs). Binding preferences of H2AX and γH2AX along the KSHV episome were examined by whole-episome ChIP analysis. We showed that γH2AX had a higher relative binding activity along the TR regions than that of the long unique region (LUR), which highlighted the importance of H2AX phosphorylation during KSHV infection. Furthermore, knockdown of H2AX resulted in decreased KSHV episome copy number. Notably, the C terminus of LANA contributed to phosphorylation of H2AX. However, phosphorylation was not dependent on the ability of LANA to drive KSHV-infected cells into S-phase. Thus, H2AX contributes to association of LANA with the TRs, and phosphorylation of H2AX is likely important for its increased density at the TRs.
Figures





Similar articles
-
Kaposi's sarcoma-associated herpesvirus induces the ATM and H2AX DNA damage response early during de novo infection of primary endothelial cells, which play roles in latency establishment.J Virol. 2014 Mar;88(5):2821-34. doi: 10.1128/JVI.03126-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352470 Free PMC article.
-
Analysis of viral cis elements conferring Kaposi's sarcoma-associated herpesvirus episome partitioning and maintenance.J Virol. 2007 Sep;81(18):9825-37. doi: 10.1128/JVI.00842-07. Epub 2007 Jul 11. J Virol. 2007. PMID: 17626102 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus LANA-Adjacent Regions with Distinct Functions in Episome Segregation or Maintenance.J Virol. 2019 Mar 5;93(6):e02158-18. doi: 10.1128/JVI.02158-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626680 Free PMC article.
-
KSHV LANA--the master regulator of KSHV latency.Viruses. 2014 Dec 11;6(12):4961-98. doi: 10.3390/v6124961. Viruses. 2014. PMID: 25514370 Free PMC article. Review.
-
The latency-associated nuclear antigen, a multifunctional protein central to Kaposi's sarcoma-associated herpesvirus latency.Future Microbiol. 2011 Dec;6(12):1399-413. doi: 10.2217/fmb.11.137. Future Microbiol. 2011. PMID: 22122438 Free PMC article. Review.
Cited by
-
Bub1 in Complex with LANA Recruits PCNA To Regulate Kaposi's Sarcoma-Associated Herpesvirus Latent Replication and DNA Translesion Synthesis.J Virol. 2015 Oct;89(20):10206-18. doi: 10.1128/JVI.01524-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223641 Free PMC article.
-
Immune escape of avian oncogenic Marek's disease herpesvirus and antagonistic host immune responses.NPJ Vaccines. 2024 Jun 15;9(1):109. doi: 10.1038/s41541-024-00905-0. NPJ Vaccines. 2024. PMID: 38879650 Free PMC article. Review.
-
Histone H2AX phosphorylation as a measure of DNA double-strand breaks and a marker of environmental stress and disease activity in lupus.Lupus Sci Med. 2016 Apr 29;3(1):e000148. doi: 10.1136/lupus-2016-000148. eCollection 2016. Lupus Sci Med. 2016. PMID: 27158526 Free PMC article.
-
Epstein-Barr Virus: Diseases Linked to Infection and Transformation.Front Microbiol. 2016 Oct 25;7:1602. doi: 10.3389/fmicb.2016.01602. eCollection 2016. Front Microbiol. 2016. PMID: 27826287 Free PMC article. Review.
-
3D culture conditions support Kaposi's sarcoma herpesvirus (KSHV) maintenance and viral spread in endothelial cells.J Mol Med (Berl). 2021 Mar;99(3):425-438. doi: 10.1007/s00109-020-02020-8. Epub 2021 Jan 23. J Mol Med (Berl). 2021. PMID: 33484281 Free PMC article.
References
-
- Weitzman MD, Carson CT, Schwartz RA, Lilley CE. 2004. Interactions of viruses with the cellular DNA repair machinery. DNA Repair (Amst.) 3:1165–1173 - PubMed
-
- Koopal S, Furuhjelm JH, Jarviluoma A, Jaamaa S, Pyakurel P, Pussinen C, Wirzenius M, Biberfeld P, Alitalo K, Laiho M, Ojala PM. 2007. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog. 3:1348–1360 doi:10.1371/journal.ppat.0030140 - DOI - PMC - PubMed
-
- Park J, Lee D, Seo T, Chung J, Choe J. 2000. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) open reading frame 36 protein is a serine protein kinase. J. Gen. Virol. 81:1067–1071 - PubMed
-
- Verma SC, Robertson ES. 2003. Molecular biology and pathogenesis of Kaposi sarcoma-associated herpesvirus. FEMS Microbiol. Lett. 222:155–163 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

