Porcine reproductive and respiratory syndrome virus Nsp1β inhibits interferon-activated JAK/STAT signal transduction by inducing karyopherin-α1 degradation
- PMID: 23449802
- PMCID: PMC3624296
- DOI: 10.1128/JVI.02643-12
Porcine reproductive and respiratory syndrome virus Nsp1β inhibits interferon-activated JAK/STAT signal transduction by inducing karyopherin-α1 degradation
Abstract
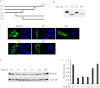
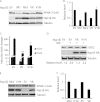
Porcine reproductive and respiratory syndrome virus (PRRSV) inhibits the interferon-mediated antiviral response. Type I interferons (IFNs) induce the expression of IFN-stimulated genes by activating phosphorylation of both signal transducer and activator of transcription 1 (STAT1) and STAT2, which form heterotrimers (interferon-stimulated gene factor 3 [ISGF3]) with interferon regulatory factor 9 (IRF9) and translocate to the nucleus. PRRSV Nsp1β blocks the nuclear translocation of the ISGF3 complex by an unknown mechanism. In this study, we discovered that Nsp1β induced the degradation of karyopherin-α1 (KPNA1, also called importin-α5), which is known to mediate the nuclear import of ISGF3. Overexpression of Nsp1β resulted in a reduction of KPNA1 levels in a dose-dependent manner, and treatment of the cells with the proteasome inhibitor MG132 restored KPNA1 levels. Furthermore, the presence of Nsp1β induced an elevation of KPNA1 ubiquitination and a shortening of its half-life. Our analysis of Nsp1β deletion constructs showed that the N-terminal domain of Nsp1β was involved in the ubiquitin-proteasomal degradation of KPNA1. A nucleotide substitution resulting in an amino acid change from valine to isoleucine at residue 19 of Nsp1β diminished its ability to induce KPNA1 degradation and to inhibit IFN-mediated signaling. Interestingly, infection of MARC-145 cells by PRRSV strains VR-2332 and VR-2385 also resulted in KPNA1 reduction, whereas infection by an avirulent strain, Ingelvac PRRS modified live virus (MLV), did not. MLV Nsp1β had no effect on KPNA1; however, a mutant with an amino acid change at residue 19 from isoleucine to valine induced KPNA1 degradation. These results indicate that Nsp1β blocks ISGF3 nuclear translocation by inducing KPNA1 degradation and that valine-19 in Nsp1β correlates with the inhibition.
Figures





References
-
- Faaberg KS, Balasuriya UB, Brinton MA, Gorbalenya AE, Leung FC-C, Nauwynck H, Snijder EJ, Stadejek T, Yang H, Yoo D. 2011. Family Arteriviridae. In King AMQ, Lefkowitz E, Adams MJ, Carstens EB. (ed), Virus taxonomy. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, London, United Kingdom
-
- Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. 2005. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 227:385–392 - PubMed
-
- Meulenberg JJ, de Meijer EJ, Moormann RJ. 1993. Subgenomic RNAs of Lelystad virus contain a conserved leader-body junction sequence. J. Gen. Virol. 74(Pt 8):1697–1701 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

