Automated detection of sclerotic metastases in the thoracolumbar spine at CT
- PMID: 23449957
- PMCID: PMC3689444
- DOI: 10.1148/radiol.13121351
Automated detection of sclerotic metastases in the thoracolumbar spine at CT
Erratum in
-
Response.Radiology. 2013 Oct;269(1):311. doi: 10.1148/radiol.13134028. Radiology. 2013. PMID: 24191350 Free PMC article. No abstract available.
Abstract
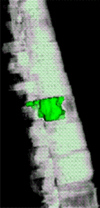
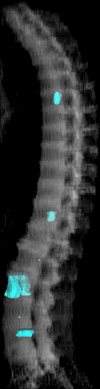
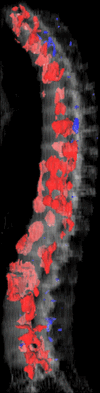
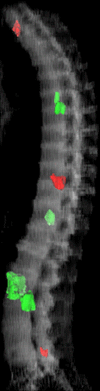
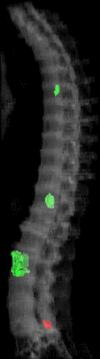
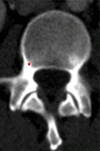
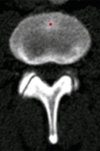
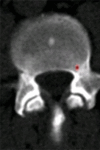
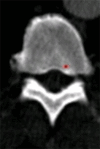
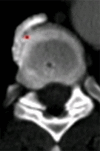
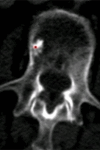
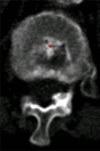
Purpose: To design and validate a computer system for automated detection and quantitative characterization of sclerotic metastases of the thoracolumbar spine on computed tomography (CT) images.
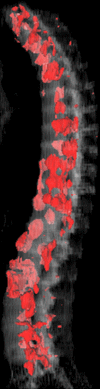
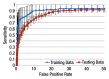
Materials and methods: This retrospective study was approved by the institutional review board and was HIPAA compliant; informed consent was waived. The data set consisted of CT examinations in 49 patients (14 female, 35 male patients; mean age, 57.0 years; range, 12-77 years), demonstrating a total of 532 sclerotic lesions of the spine of greater than 0.3 cm(3) in volume, and in 10 control case patients (four women, six men; mean age, 55.2 years; range, 19-70 years) without spinal lesions. CT examinations were divided into training and test sets, and images were analyzed according to prototypical fully-automated computer-aided detection (CAD) software. Free-response receiver operating characteristic analysis was performed.
Results: Lesion detection sensitivity on images in the training set was 90%, relative to reference-standard marked lesions (95% confidence interval [CI]: 83%, 97%), at a false-positive rate (FPR) of 10.8 per patient (95% CI: 6.6, 15.0). For images in the testing set, sensitivity was 79% (95% CI: 74%, 84%), with an FPR of 10.9 per patient (95% CI: 8.5, 13.3). False-negative findings were most commonly (37 [40%] of 93) a result of endplate proximity, with 32 (34% of 93) caused by low CT attenuation. Marginal sclerosis caused by degenerative change (174 [28.1%] of 620 actual detections) was the most common cause of false-positive detections, followed by partial volume averaging with vertebral endplates (173 [27.9%] of 620) and pedicle cortex parallel to the axial imaging plane (121 [19.5%] 620).
Conclusion: This CAD system successfully identified and segmented sclerotic lesions in the thoracolumbar spine.
Figures

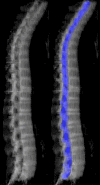
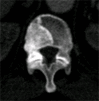


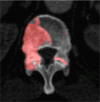



Similar articles
-
Progress in Fully Automated Abdominal CT Interpretation.AJR Am J Roentgenol. 2016 Jul;207(1):67-79. doi: 10.2214/AJR.15.15996. Epub 2016 Apr 21. AJR Am J Roentgenol. 2016. PMID: 27101207 Free PMC article. Review.
-
Lytic metastases in thoracolumbar spine: computer-aided detection at CT--preliminary study.Radiology. 2007 Mar;242(3):811-6. doi: 10.1148/radiol.2423060260. Radiology. 2007. PMID: 17325068
-
Automated Detection, Localization, and Classification of Traumatic Vertebral Body Fractures in the Thoracic and Lumbar Spine at CT.Radiology. 2016 Jan;278(1):64-73. doi: 10.1148/radiol.2015142346. Epub 2015 Jul 14. Radiology. 2016. PMID: 26172532 Free PMC article.
-
Vertebral Body Compression Fractures and Bone Density: Automated Detection and Classification on CT Images.Radiology. 2017 Sep;284(3):788-797. doi: 10.1148/radiol.2017162100. Epub 2017 Mar 16. Radiology. 2017. PMID: 28301777 Free PMC article.
-
CT temporal subtraction method for detection of sclerotic bone metastasis in the thoracolumbar spine.Eur J Radiol. 2018 Oct;107:54-59. doi: 10.1016/j.ejrad.2018.07.017. Epub 2018 Jul 26. Eur J Radiol. 2018. PMID: 30292273
Cited by
-
Cortical shell unwrapping for vertebral body abnormality detection on computed tomography.Comput Med Imaging Graph. 2014 Oct;38(7):628-38. doi: 10.1016/j.compmedimag.2014.04.001. Epub 2014 Apr 13. Comput Med Imaging Graph. 2014. PMID: 24815367 Free PMC article.
-
Mixed spine metastasis detection through positron emission tomography/computed tomography synthesis and multiclassifier.J Med Imaging (Bellingham). 2017 Apr;4(2):024504. doi: 10.1117/1.JMI.4.2.024504. Epub 2017 Jun 5. J Med Imaging (Bellingham). 2017. PMID: 28612036 Free PMC article.
-
Progress in Fully Automated Abdominal CT Interpretation.AJR Am J Roentgenol. 2016 Jul;207(1):67-79. doi: 10.2214/AJR.15.15996. Epub 2016 Apr 21. AJR Am J Roentgenol. 2016. PMID: 27101207 Free PMC article. Review.
-
Application of Artificial Intelligence Methods for Imaging of Spinal Metastasis.Cancers (Basel). 2022 Aug 20;14(16):4025. doi: 10.3390/cancers14164025. Cancers (Basel). 2022. PMID: 36011018 Free PMC article. Review.
-
Improving Computer-Aided Detection Using Convolutional Neural Networks and Random View Aggregation.IEEE Trans Med Imaging. 2016 May;35(5):1170-81. doi: 10.1109/TMI.2015.2482920. Epub 2015 Sep 28. IEEE Trans Med Imaging. 2016. PMID: 26441412 Free PMC article.
References
-
- Doi K. Overview on research and development of computer-aided diagnostic schemes. Semin Ultrasound CT MR 2004;25(5):404–410 - PubMed
-
- Morton MJ, Whaley DH, Brandt KR, Amrami KK. Screening mammograms: interpretation with computer-aided detection—prospective evaluation. Radiology 2006;239(2):375–383 - PubMed
-
- Timp S, Varela C, Karssemeijer N. Computer-aided diagnosis with temporal analysis to improve radiologists’ interpretation of mammographic mass lesions. IEEE Trans Inf Technol Biomed 2010;14(3):803–808 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

