Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1)
- PMID: 23449980
- PMCID: PMC3617280
- DOI: 10.1074/jbc.M112.438804
Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1)
Abstract
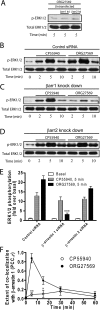
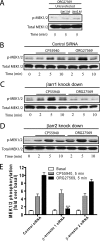
The cannabinoid receptor 1 (CB1) is a G protein-coupled receptor primarily expressed in brain tissue that has been implicated in several disease states. CB1 allosteric compounds, such as ORG27569, offer enormous potential as drugs over orthosteric ligands, but their mechanistic, structural, and downstream effects upon receptor binding have not been established. Previously, we showed that ORG27569 enhances agonist binding affinity to CB1 but inhibits G protein-dependent agonist signaling efficacy in HEK293 cells and rat brain expressing the CB1 receptor (Ahn, K. H., Mahmoud, M. M., and Kendall, D. A. (2012) J. Biol. Chem. 287, 12070-12082). Here, we identify the mediators of CB1 receptor internalization and ORG27569-induced G protein-independent signaling. Using siRNA technology, we elucidate an ORG27569-induced signaling mechanism for CB1 wherein β-arrestin 1 mediates short term signaling to ERK1/2 with a peak at 5 min and other upstream kinase components including MEK1/2 and c-Src. Consistent with these findings, we demonstrate co-localization of CB1-GFP with red fluorescent protein-β-arrestin 1 upon ORG27569 treatment using confocal microscopy. In contrast, we show the critical role of β-arrestin 2 in CB1 receptor internalization upon treatment with CP55940 (agonist) or treatment with ORG27569. These results demonstrate for the first time the involvement of β-arrestin in CB1-biased signaling by a CB1 allosteric modulator and also define the differential role of the two β-arrestin isoforms in CB1 signaling and internalization.
Figures


References
-
- Price M. R., Baillie G. L., Thomas A., Stevenson L. A., Easson M., Goodwin R., McLean A., McIntosh L., Goodwin G., Walker G., Westwood P., Marrs J., Thomson F., Cowley P., Christopoulos A., Pertwee R. G., Ross R. A. (2005) Allosteric modulation of the cannabinoid CB1 receptor. Mol. Pharmacol. 68, 1484–1495 - PubMed
-
- Zhang Y., Rodriguez A. L., Conn P. J. (2005) Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J. Pharmacol. Exp. Ther. 315, 1212–1219 - PubMed
-
- Knudsen L. B., Kiel D., Teng M., Behrens C., Bhumralkar D., Kodra J. T., Holst J. J., Jeppesen C. B., Johnson M. D., de Jong J. C., Jorgensen A. S., Kercher T., Kostrowicki J., Madsen P., Olesen P. H., Petersen J. S., Poulsen F., Sidelmann U. G., Sturis J., Truesdale L., May J., Lau J. (2007) Small-molecule agonists for the glucagon-like peptide 1 receptor. Proc. Natl. Acad. Sci. U.S.A. 104, 937–942 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

