Medium-chain fatty acid-sensing receptor, GPR84, is a proinflammatory receptor
- PMID: 23449982
- PMCID: PMC3624448
- DOI: 10.1074/jbc.M112.420042
Medium-chain fatty acid-sensing receptor, GPR84, is a proinflammatory receptor
Abstract
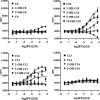
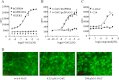
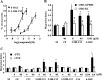
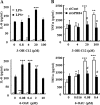
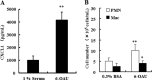
G protein-coupled receptor 84 (GPR84) is a putative receptor for medium-chain fatty acids (MCFAs), whose pathophysiological roles have not yet been clarified. Here, we show that GPR84 was activated by MCFAs with the hydroxyl group at the 2- or 3-position more effectively than nonhydroxylated MCFAs. We also identified a surrogate agonist, 6-n-octylaminouracil (6-OAU), for GPR84. These potential ligands and the surrogate agonist, 6-OAU, stimulated [(35)S]GTP binding and accumulated phosphoinositides in a GPR84-dependent manner. The surrogate agonist, 6-OAU, internalized GPR84-EGFP from the cell surface. Both the potential ligands and 6-OAU elicited chemotaxis of human polymorphonuclear leukocytes (PMNs) and macrophages and amplified LPS-stimulated production of the proinflammatory cytokine IL-8 from PMNs and TNFα from macrophages. Furthermore, the intravenous injection of 6-OAU raised the blood CXCL1 level in rats, and the inoculation of 6-OAU into the rat air pouch accumulated PMNs and macrophages in the site. Our results indicate a proinflammatory role of GPR84, suggesting that the receptor may be a novel target to treat chronic low grade inflammation associated-disease.
Figures

References
-
- Wellendorph P., Johansen L. D., Bräuner-Osborne H. (2009) Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol. Pharmacol. 76, 453–465 - PubMed
-
- Blad C. C., Tang C., Offermanns S. (2012) G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov. 11, 603–619 - PubMed
-
- Briscoe C. P., Tadayyon M., Andrews J. L., Benson W. G., Chambers J. K., Eilert M. M., Ellis C., Elshourbagy N. A., Goetz A. S., Minnick D. T., Murdock P. R., Sauls H. R., Jr., Shabon U., Spinage L. D., Strum J. C., Szekeres P. G., Tan K. B., Way J. M., Ignar D. M., Wilson S., Muir A. I. (2003) The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278, 11303–11311 - PubMed
-
- Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., Tanaka H., Maruyama M., Satoh R., Okubo S., Kizawa H., Komatsu H., Matsumura F., Noguchi Y., Shinohara T., Hinuma S., Fujisawa Y., Fujino M. (2003) Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422, 173–176 - PubMed
-
- Overton H. A., Babbs A. J., Doel S. M., Fyfe M. C., Gardner L. S., Griffin G., Jackson H. C., Procter M. J., Rasamison C. M., Tang-Christensen M., Widdowson P. S., Williams G. M., Reynet C. (2006) Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 3, 167–175 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

