Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients
- PMID: 23450558
- PMCID: PMC6823220
- DOI: 10.1002/14651858.CD005133.pub3
Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients
Update in
-
Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients.Cochrane Database Syst Rev. 2025 Jan 14;1(1):CD005133. doi: 10.1002/14651858.CD005133.pub4. Cochrane Database Syst Rev. 2025. PMID: 39807668
Abstract
Background: Cytomegalovirus (CMV) is a significant cause of morbidity and mortality in solid organ transplant recipients. Pre-emptive treatment of patients with CMV viraemia using antiviral agents has been suggested as an alternative to routine prophylaxis to prevent CMV disease. This is an update of a Cochrane review first published in 2005.
Objectives: This review was conducted to evaluate the efficacy of pre-emptive treatment with antiviral medications in preventing symptomatic CMV disease.
Search methods: For this update, we searched the Cochrane Renal Group's Specialised Register (to 16 January 2013) through contact with the Trials' Search Co-ordinator using search terms relevant to this review.
Selection criteria: We included randomised controlled trials (RCTs) of pre-emptive treatment compared with placebo, no specific treatment or with antiviral prophylaxis in solid organ transplant recipients.
Data collection and analysis: Four authors assessed the quality and extracted all data. Analyses used a random-effects model and results were expressed as risk ratio (RR) and 95% confidence intervals (CI).
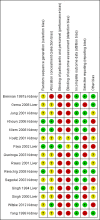
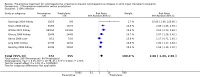
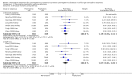
Main results: We identified 15 eligible studies (1098 participants). Of these, six investigated pre-emptive treatment versus placebo or treatment of CMV when disease occurred (standard care), eight looked at pre-emptive treatment versus antiviral prophylaxis, and one reported on oral versus intravenous pre-emptive treatment.Assessment of risk of bias identified that the processes reported for sequence generation and allocation concealment were at low risk of bias in only five and three studies, respectively. All studies were considered to be at low risk of attrition bias, and seven studies were considered to be at low risk of bias for selective reporting. Only one study reported adequate blinding of participants and personnel; no study reported blinding of outcome assessment.Compared with placebo or standard care, pre-emptive treatment significantly reduced the risk of CMV disease (6 studies, 288 participants: RR 0.29, 95% CI 0.11 to 0.80) but not acute rejection (3 studies, 185 participants: RR 1.21, 95% CI 0.69 to 2.12) or all-cause mortality (3 studies, 176 participants: RR 1.23, 95% CI 0.35 to 4.30). Comparative studies of pre-emptive therapy versus prophylaxis showed no significant differences in preventing CMV disease between pre-emptive and prophylactic therapy (7 studies, 753 participants: RR 1.00, 95% CI 0.36 to 2.74) but there was significant heterogeneity (I² = 63%). Leucopenia was significantly less common with pre-emptive therapy compared with prophylaxis (6 studies, 729 participants: RR 0.42, 95% CI 0.20 to 0.90). Other adverse effects did not differ significantly or were not reported. There were no significant differences in the risks of all-cause mortality, graft loss, acute rejection and infections other than CMV.
Authors' conclusions: Few RCTs have evaluated the effects of pre-emptive therapy to prevent CMV disease. Pre-emptive therapy is effective compared with placebo or standard care. Despite the inclusion of five additional studies in this update, the efficacy of pre-emptive therapy compared with prophylaxis to prevent CMV disease remains unclear due to significant heterogeneity between studies. Additional head-to-head studies are required to determine the relative benefits and harms of pre-emptive therapy and prophylaxis to prevent CMV disease in solid organ transplant recipients.
Conflict of interest statement
None known.
Figures

















Update of
-
Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients.Cochrane Database Syst Rev. 2006 Jan 25;(1):CD005133. doi: 10.1002/14651858.CD005133.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2013 Feb 28;(2):CD005133. doi: 10.1002/14651858.CD005133.pub3. PMID: 16437521 Updated.
References
References to studies included in this review
-
- Brennan DC, Garlock KA, Lippmann BA, Buller RS, Gaudreault‐Keener M, Lowell JA, et al. Control of cytomegalovirus‐associated morbidity in renal transplant patients using intensive monitoring and either preemptive or deferred therapy. Journal of the American Society of Nephrology 1997;8(1):118‐25. [MEDLINE: ] - PubMed
- Brennan DC, Garlock KA, Lippmann BJ, Buller RS, Gaudreault‐Keener M, Lowell JA, et al. Polymerase chain reaction‐triggered preemptive or deferred therapy to control cytomegalovirus‐associated morbidity and costs in renal transplant patients. Transplantation Proceedings 1997;29(1‐2):809‐11. [MEDLINE: ] - PubMed
- Brennan DC, Garlock KA, Lippmann BJ, Buller RS, Gaudreault‐Keener M, Lowell JA, et al. The prevalence of human herpesvirus‐7 in renal transplant recipients is unaffected by oral or intravenous ganciclovir. Journal of Infectious Diseases 2000;18(5):1557‐61. [MEDLINE: ] - PubMed
-
- Gerna G, Lilleri D, Callegaro A, Goglio A, Cortese S, Stroppa P, et al. Prophylaxis followed by preemptive therapy versus preemptive therapy for prevention of human cytomegalovirus disease in pediatric patients undergoing liver transplantation. Transplantation 2008;86(1):163‐6. [MEDLINE: ] - PubMed
-
- Jung C, Engelmann E, Borner K, Offermann G. Preemptive oral ganciclovir therapy versus prophylaxis to prevent symptomatic cytomegalovirus infection after kidney transplantation. Transplantation Proceedings 2001;33(7‐8):3621‐3. [MEDLINE: ] - PubMed
- Offermann G, Jung C. Preemptive oral ganciclovir therapy versus prophylaxis to prevent symptomatic cytomegalovirus infection after kidney transplantation [abstract no: 1094]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. - PubMed
-
- Brennan DC, Hardinger KL, Bohl DL, Lockwood M, Torrence S, Schuessler R, et al. Preemptive vs prophylactic valganciclovir for CMV in renal transplantation: early results from a randomized, prospective trial. [abstract]. Journal of the American Society of Nephrology 2004;15(Oct):23A.
- Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. American Journal of Transplantation 2006;6(9):2134‐43. [MEDLINE: ] - PubMed
- Spinner ML, Saab G, Casabar E, Bowman LJ, Storch GA, Brennan DC. Impact of prophylactic versus preemptive valganciclovir on long‐term renal allograft outcomes. Transplantation 2010;90(4):412‐8. [MEDLINE: ] - PMC - PubMed
-
- Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in long‐term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. American Journal of Transplantation 2008;8(5):975‐83. [MEDLINE: ] - PubMed
- Radermacher J, Fricke L, Burg M, Mischak H, Rohde F, Kliem V. Influence of prophylactic compared with early ganciclovir treatment on graft survival in renal allograft recipients. [abstract]. Journal of the American Society of Nephrology 2006;17(Abstracts):111A.
References to studies excluded from this review
-
- Ahsan N, Holman MJ, Dhilon S, Ream L, Yang HC. Oral ganciclovir (CytoveneR) effectively prevents cytomegalovirus (CMV) infection in renal transplant patients [abstract]. Journal of the American Society of Nephrology 1996;7(9):1929.
- Ahsan N, Holman MJ, Yang HC. Efficacy of oral ganciclovir in prevention of cytomegalovirus infection in post‐kidney transplant patients. Clinical Transplantation 1997;11(6):633‐9. [MEDLINE: ] - PubMed
- Ahsan N, Holman MJ, Yang HC. Oral ganciclovir is effective in preventing CMV infection in renal transplant recipients. [abstract]. Nephrology 1997;3(Suppl 1):S70.
-
- Ahsan N, Holman MJ, Sonderbye L, Langhoff E, Yang HC. Oral ganciclovir in the prevention of cytomegalovirus infection in post kidney transplant "CMV at risk" recipients: a controlled, comparative study of two regimens (750 mg Bid and 500 mg Bid). Transplantation Proceedings 1998;30(4):1383‐5. [MEDLINE: ] - PubMed
-
- Arbo MD, Snydman DR, Wong JB, Goldberg HS, Schmid CH, Pauker SG. Cytomegalovirus immune globulin after liver transplantation: a cost‐effectiveness analysis. Clinical Transplantation 2000;14(1):19‐27. [MEDLINE: ] - PubMed
-
- Badley AD, Seaberg EC, Porayko MK, Wiesner RH, Keating MR, Wilhelm MP, et al. Prophylaxis of cytomegalovirus infection in liver transplantation: A randomized trial comparing a combination of ganciclovir and acyclovir to acyclovir. Transplantation 1997;64(1):66‐73. [MEDLINE: ] - PubMed
- Paya CV, Marin E, Keating M, Dickson R, Porayko M, Wiesner R. Solid organ transplantation: results and implications of acyclovir use in liver transplants. Journal of Medical Virology 1993;Suppl 1:123‐7. [MEDLINE: ] - PubMed
-
- Balfour HH. Prevention of cytomegalovirus disease in renal allograft recipients. Scandinavian Journal of Infectious Diseases ‐ Supplement 1991;80:88‐93. [MEDLINE: ] - PubMed
- Balfour HH, Chace BA, Stapleton JT, Simmons RL, Fryd DS. A randomized, placebo‐controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts. New England Journal of Medicine 1989;320(21):1381‐7. [MEDLINE: ] - PubMed
- Balfour HH, Fletcher CV, Dunn D. Prevention of cytomegalovirus disease with oral acyclovir. Transplantation Proceedings 1991;23(2 Suppl 1):17‐9. [MEDLINE: ] - PubMed
- Balfour Jr HH, Bean B, Mitchell CD. Acyclovir in immunocompromised patients with cytomegalovirus disease. A controlled trial at one institution. American Journal of Medicine 1982;73(1A):241‐8. [MEDLINE: ] - PubMed
- Fletcher CV, Englund JA, Edelman CK, Gross CR, Dunn DL, Balfour HH. Pharmacologic basis for high‐dose oral acyclovir prophylaxis of cytomegalovirus disease in renal allograft recipients. Antimicrobial Agents & Chemotherapy 1991;35(5):938‐43. [MEDLINE: ] - PMC - PubMed
References to studies awaiting assessment
-
- Scott GM, Naing Z, Pavlovic J, Iwasenko JM, Angus P, Jones R, et al. Viral factors influencing the outcome of human cytomegalovirus infection in liver transplant recipients. Journal of Clinical Virology 2011;51(4):229‐33. [MEDLINE: ] - PubMed
References to ongoing studies
-
- Hoffmann‐La Roche. A study of Valcyte (valganciclovir) CMV prophylaxis after renal transplantation. http://clinicaltrials.gov/ct2/show/NCT00372229 (accessed 10 January 2013).
-
- Potena L, Grigioni F. Prevention of Transplant Atherosclerosis With Everolimus and Anti‐cytomegalovirus Therapy (PROTECT). http://clinicaltrials.gov/ct2/show/NCT00966836 (accessed 10 January 2013).
-
- Singh N. Prophylaxis Versus Preemptive Therapy for the Prevention of CMV in Liver Transplant Recipients (CAPSIL). http://clinicaltrials.gov/ct2/show/NCT01552369 (accessed 10 Janaury 2013).
Additional references
-
- Arthurs SK, Eid AJ, Pedersen RA, Dierkhising RA, Kremers WK, Patel R, et al. Delayed‐onset primary cytomegalovirus disease after liver transplantation. Liver Transplantation 2007;13(12):1703‐9. [MEDLINE: ] - PubMed
-
- Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, et al. Delayed‐onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clinical Infectious Diseases 2008;46(6):840‐6. [MEDLINE: ] - PubMed
-
- British Transplantation Society. Guidelines for the prevention and management of cytomegalovirus disease after solid organ transplantation. 3rd Edition. London: British Transplantation Society, 2011. - PubMed
-
- Pillmore H. Pre‐emptive treatment of cytomegalovirus. http://www.cari.org.au/TRANS_cmv_published/Pre‐emptive_treatment_CMV_Oct... (accessed 10 January 2013).
-
- European Best Practice Guidelines Expert Group on Renal Transplantation. Section III: The transplant recipient from initial transplant hospitalization to 1 year post transplant. Nephrology Dialysis Transplantation 2000;15(Suppl 7):52‐85.
References to other published versions of this review
-
- Strippoli GF, Hodson EM, Jones C, Craig JC. Preemptive treatment for cytomegalovirus viremia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation 2006;81(2):139‐45. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

