Epigenetic regulation of NK cell differentiation and effector functions
- PMID: 23450696
- PMCID: PMC3584244
- DOI: 10.3389/fimmu.2013.00055
Epigenetic regulation of NK cell differentiation and effector functions
Abstract
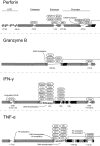
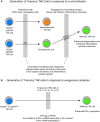
Upon maturation, natural killer (NK) cells acquire effector functions and regulatory receptors. New insights suggest a considerable functional heterogeneity and dynamic regulation of receptor expression in mature human NK cell subsets based on different developmental axes. Such processes include acquisition of lytic granules as well as regulation of cytokine production in response to exogenous cytokine stimulation or target cell interactions. One axis is regulated by expression of inhibitory receptors for self-MHC class I molecules, whereas other axes are less well defined but likely are driven by different activating receptor engagements or cytokines. Moreover, the recent identification of long-lived NK cell subsets in mice that are able to expand and respond rapidly following a secondary viral challenge suggest previously unappreciated plasticity in the programming of NK cell differentiation. Here, we review advances in our understanding of mature NK cell development and plasticity with regards to regulation of cellular function. Furthermore, we highlight some of the major questions that remain pertaining to the epigenetic changes that underlie the differentiation and functional specialization of NK cells and the regulation of their responses.
Keywords: NK cell; development; epigenetics; memory; transcription factors.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

