Development in Aspergillus
- PMID: 23450714
- PMCID: PMC3563288
- DOI: 10.3114/sim0006
Development in Aspergillus
Abstract
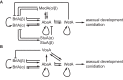
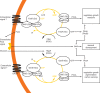
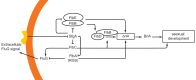
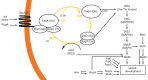
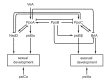
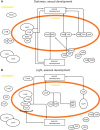
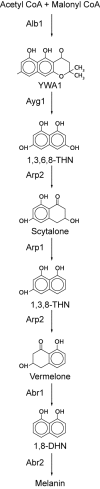
The genus Aspergillus represents a diverse group of fungi that are among the most abundant fungi in the world. Germination of a spore can lead to a vegetative mycelium that colonizes a substrate. The hyphae within the mycelium are highly heterogeneous with respect to gene expression, growth, and secretion. Aspergilli can reproduce both asexually and sexually. To this end, conidiophores and ascocarps are produced that form conidia and ascospores, respectively. This review describes the molecular mechanisms underlying growth and development of Aspergillus.
Keywords: Aspergillus; ascocarp; ascospore; asexual reproduction; conidiophore; conidium; development; fruiting body; fungi; heterogeneity; sexual reproduction; vegetative mycelium.
Figures



References
-
- Adams TH, Boylan MT, Timberlake WE. (1988). brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54: 353–362 - PubMed
-
- Agnihotri VP. (1964). Studies on Aspergilli. XVI. Effect of pH, temperature, and carbon and nitrogen interaction. Mycopathologia et Mycologia Applicata 24: 305–314 - PubMed
-
- Aguirre J. (1993). Spatial and temporal controls of the Aspergillus brlA developmental regulatory gene. Molecular Microbiology 8: 211–8 - PubMed
-
- Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, et al. (2009). Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460: 1117–1121 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
