Safety and immunogenicity of neonatal pneumococcal conjugate vaccination in Papua New Guinean children: a randomised controlled trial
- PMID: 23451070
- PMCID: PMC3579820
- DOI: 10.1371/journal.pone.0056698
Safety and immunogenicity of neonatal pneumococcal conjugate vaccination in Papua New Guinean children: a randomised controlled trial
Abstract
Background: Approximately 826,000 children, mostly young infants, die annually from invasive pneumococcal disease. A 6-10-14-week schedule of pneumococcal conjugate vaccine (PCV) is efficacious but neonatal PCV may provide earlier protection and better coverage. We conducted an open randomized controlled trial in Papua New Guinea to compare safety, immunogenicity and priming for memory of 7-valent PCV (PCV7) given in a 0-1-2-month (neonatal) schedule with that of the routine 1-2-3-month (infant) schedule.
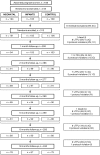
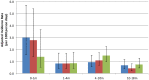
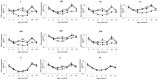
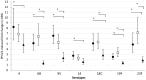
Methods: We randomized 318 infants at birth to receive PCV7 in the neonatal or infant schedule or no PCV7. All infants received 23-valent pneumococcal polysaccharide vaccine (PPV) at age 9 months. Serotype-specific serum IgG for PCV7 (VT) serotypes and non-VT serotypes 2, 5 and 7F were measured at birth and 2, 3, 4, 9, 10 and 18 months of age. Primary outcomes were geometric mean concentrations (GMCs) and proportions with concentration ≥ 0.35 µg/ml of VT serotype-specific pneumococcal IgG at age 2 months and one month post-PPV.
Results: We enrolled 101, 105 and 106 infants, respectively, into neonatal, infant and control groups. Despite high background levels of maternally derived antibody, both PCV7 groups had higher GMCs than controls at age 2 months for serotypes 4 (p<0.001) and 9V (p<0.05) and at age 3 months for all VTs except 6B. GMCs for serotypes 4, 9V, 18C and 19F were significantly higher (p<0.001) at age 2 months in the neonatal (one month post-dose2 PCV7) than in the infant group (one month post-dose1 PCV7). PPV induced significantly higher VT antibody responses in PCV7-primed than unprimed infants, with neonatal and infant groups equivalent. High VT and non-VT antibody concentrations generally persisted to age 18 months.
Conclusions: PCV7 is well-tolerated and immunogenic in PNG neonates and young infants and induces immunologic memory to PPV booster at age 9 months with antibody levels maintained to age 18 months.
Trial registration: ClinicalTrials.gov NCT00219401.
Conflict of interest statement
Figures
References
-
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, et al. (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374: 893–902. - PubMed
-
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. (2003) Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 348: 1737–1746. - PubMed
-
- Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, et al. (2003) A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 349: 1341–1348. - PubMed
-
- Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, et al. (2005) Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365: 1139–1146. - PubMed
-
- O’Brien KL, Moulton LH, Reid R, Weatherholtz R, Oski J, et al. (2003) Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362: 355–361. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

