Antineoplastic effects of α-santalol on estrogen receptor-positive and estrogen receptor-negative breast cancer cells through cell cycle arrest at G2/M phase and induction of apoptosis
- PMID: 23451128
- PMCID: PMC3579946
- DOI: 10.1371/journal.pone.0056982
Antineoplastic effects of α-santalol on estrogen receptor-positive and estrogen receptor-negative breast cancer cells through cell cycle arrest at G2/M phase and induction of apoptosis
Erratum in
- PLoS One. 2013;8(6). doi:10.1371/annotation/c732480c-eb97-4eff-acb6-300797f4efa9
Abstract
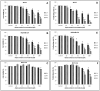
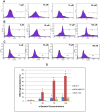
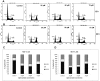
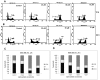
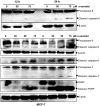
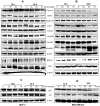
Anticancer efficacy and the mechanism of action of α-santalol, a terpenoid isolated from sandalwood oil, were investigated in human breast cancer cells by using p53 wild-type MCF-7 cells as a model for estrogen receptor (ER)-positive and p53 mutated MDA-MB-231 cells as a model for ER-negative breast cancer. α-Santalol inhibited cell viability and proliferation in a concentration and time-dependent manner in both cells regardless of their ER and/or p53 status. However, α-santalol produced relatively less toxic effect on normal breast epithelial cell line, MCF-10A. It induced G2/M cell cycle arrest and apoptosis in both MCF-7 and MDA-MB-231 cells. Cell cycle arrest induced by α-santalol was associated with changes in the protein levels of BRCA1, Chk1, G2/M regulatory cyclins, Cyclin dependent kinases (CDKs), Cell division cycle 25B (Cdc25B), Cdc25C and Ser-216 phosphorylation of Cdc25C. An up-regulated expression of CDK inhibitor p21 along with suppressed expression of mutated p53 was observed in MDA-MB-231 cells treated with α-santalol. On the contrary, α-santalol did not increase the expression of wild-type p53 and p21 in MCF-7 cells. In addition, α-santalol induced extrinsic and intrinsic pathways of apoptosis in both cells with activation of caspase-8 and caspase-9. It led to the activation of the executioner caspase-6 and caspase-7 in α-santalol-treated MCF-7 cells and caspase-3 and caspase-6 in MDA-MB-231 cells along with strong cleavage of poly(ADP-ribose) polymerase (PARP) in both cells. Taken together, this study for the first time identified strong anti-neoplastic effects of α-santalol against both ER-positive and ER-negative breast cancer cells.
Conflict of interest statement
Figures

References
-
- Zhang X, Dwivedi C (2011) Skin cancer chemoprevention by α-santalol. Front Biosci (Schol Ed) 3: 777–787. - PubMed
-
- Burdock GA, Carabin IG (2008) Safety assessment of sandalwood oil (Santalum album L.) Food Chem Toxicol. 46: 421–432. - PubMed
-
- Dwivedi C, Guan X, Harmsen WL, Voss AL, Goetz-Parten DE, et al. (2003) Chemopreventive effects of α-santalol on skin tumor development in CD-1 and SENCAR mice. Cancer Epidemiol Biomarkers Prev 12: 151–156. - PubMed
-
- Dwivedi C, Valluri HB, Guan X, Agarwal R (2006) Chemopreventive effects of α-santalol on ultraviolet B radiation-induced skin tumor development in SKH-1 hairless mice. Carcinogenesis 27: 1917–1922. - PubMed
-
- Kaur M, Agarwal C, Singh RP, Dwivedi C, Agarwal R (2005) Skin cancer chemopreventive agent, α-santalol, induces apoptotic death of human epidermoid carcinoma A431 cells via caspase activation together with dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis 26: 369–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

