VPO1 mediates ApoE oxidation and impairs the clearance of plasma lipids
- PMID: 23451244
- PMCID: PMC3581477
- DOI: 10.1371/journal.pone.0057571
VPO1 mediates ApoE oxidation and impairs the clearance of plasma lipids
Abstract
Objective: ApoE is an abundant component of chylomicron, VLDL, IDL, and HDL. It binds to multiple types of lipids and is implicated in cholesterol and triglyceride homeostasis. Oxidation of ApoE plays a crucial role in the genesis of atherosclerosis. It is proposed that heme-containing peroxidases (hPx) are major mediators of lipoprotein oxidization. Vascular peroxidase 1 (VPO1) is a recently-discovered hPx, which is expressed in cardiovascular system, lung, liver etc. and secreted into plasma. Its plasma concentration is three orders of magnitude of that of myeloperoxidase. If VPO1 mediates ApoE oxidation and affects the lipid metabolism remains to be elucidated.
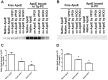
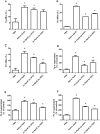
Methods: Recombinant ApoE and VPO1 were expressed and purified from stably-expressing cell lines deriving from HEK293 cells. ApoE oxidation was carried out by VPO1 in the presence of H2O2 and chloride. ApoE oxidation was verified by a variety of approaches including immunoblot and amino acid analyses. To evaluate the functional changes in VPO1-oxidized ApoE, lipid emulsion particle binding assays were employed.
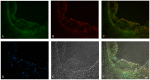
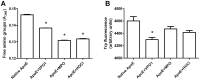
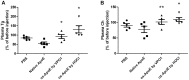
Results: Oxidized ApoE binds weaker to lipid emulsion particles, which mimic the large lipid complexes in vivo. In lipid efflux assay, oxidized ApoE showed reduced capability in efflux of lipids from foam cells. Mice administrated with oxidized ApoE via blood exhibited weaker clearance ability of plasma lipids.
Conclusions: Our data suggest that VPO1 is a new mediator regulating lipid homeostasis, implying a role in genesis and development of atherosclerosis.
Conflict of interest statement
Figures

References
-
- Choy PC, Siow YL, Mymin D, Karmin O (2004) Lipids and atherosclerosis. Biochem Cell Biol 82: 212–224. - PubMed
-
- Cox RA, Garcia-Palmieri MR (1990) Cholesterol, Triglycerides, and Associated Lipoproteins. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston.
-
- Mahley RW, Innerarity TL, Rall SC Jr, Weisgraber KH (1984) Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res 25: 1277–1294. - PubMed
-
- Mahley RW (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240: 622–630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

