Making the diagnosis of frontotemporal lobar degeneration
- PMID: 23451743
- PMCID: PMC3629720
- DOI: 10.5858/arpa.2012-0075-RA
Making the diagnosis of frontotemporal lobar degeneration
Abstract
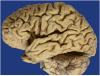
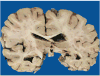
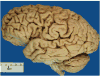
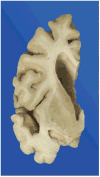
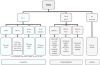
Context: Autopsy evaluation of the brain of a patient with frontotemporal dementia (FTD) can be daunting to the general pathologist. At some point in their training, most pathologists learn about Pick disease, and can recognize Pick bodies, the morphologic hallmark of Pick disease. Pick disease is a type of frontotemporal lobar degeneration (FTLD), the general category of pathologic process underlying most cases of FTD. The 2 major categories of pathologic FTLD are tauopathies (FTLD-tau) and ubiquitinopathies (FTLD-U). Pick disease is one of the FTLD-tau subtypes and is termed FTLD-tau (PiD).
Objective: To "demystify" FTLDs, and to demonstrate that subtypes of FTLD-tau and FTLD-U can be easily determined by following a logical, stepwise, histochemical, and immunohistochemical investigation of the FTD autopsy brain.
Data sources: Previously published peer-reviewed articles.
Conclusions: The hope is that this article will be a useful reference for the general pathologist faced with performing a brain autopsy on a decedent with frontotemporal dementia.
Conflict of interest statement
The author has no relevant financial interest in the products or companies described in this article.
Figures

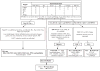
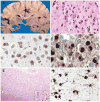

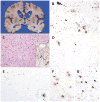
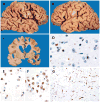
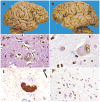
References
-
- Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer’s disease: a primer for practicing pathologists. Arch Pathol Lab Med. 1993;117(2):132–144. - PubMed
-
- Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), II: standardization of neuropathological assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. - PubMed
-
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095–1097. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

