The underappreciated role of allostery in the cellular network
- PMID: 23451894
- PMCID: PMC6407633
- DOI: 10.1146/annurev-biophys-083012-130257
The underappreciated role of allostery in the cellular network
Abstract
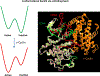
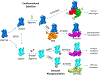
Allosteric propagation results in communication between distinct sites in the protein structure; it also encodes specific effects on cellular pathways, and in this way it shapes cellular response. One example of long-range effects is binding of morphogens to cell surface receptors, which initiates a cascade of protein interactions that leads to genome activation and specific cellular action. Allosteric propagation results from combinations of multiple factors, takes place through dynamic shifts of conformational ensembles, and affects the equilibria of macromolecular interactions. Here, we (a) emphasize the well-known yet still underappreciated role of allostery in conveying explicit signals across large multimolecular assemblies and distances to specify cellular action; (b) stress the need for quantitation of the allosteric effects; and finally, (c) propose that each specific combination of allosteric effectors along the pathway spells a distinct function. The challenges are colossal; the inspiring reward will be predicting function, misfunction, and outcomes of drug regimes.
Figures


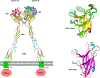

References
-
- Antal MA, Bode C, Csermely P. 2009. Perturbation waves in proteins and protein networks: applications of percolation and game theories in signaling and drug design. Current protein & peptide science 10:161–72 - PubMed
-
- Bagowski CP, Ferrell JE Jr. 2001. Bistability in the JNK cascade. Current biology: CB 11:1176–82 - PubMed
-
- Bhalla US, Ram PT, Iyengar R. 2002. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science 297:1018–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

