The multiple roles of histidine in protein interactions
- PMID: 23452343
- PMCID: PMC3599372
- DOI: 10.1186/1752-153X-7-44
The multiple roles of histidine in protein interactions
Abstract
Background: Among the 20 natural amino acids histidine is the most active and versatile member that plays the multiple roles in protein interactions, often the key residue in enzyme catalytic reactions. A theoretical and comprehensive study on the structural features and interaction properties of histidine is certainly helpful.
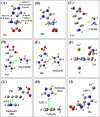
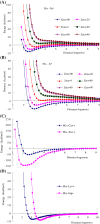
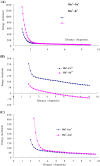
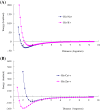
Results: Four interaction types of histidine are quantitatively calculated, including: (1) Cation-π interactions, in which the histidine acts as the aromatic π-motif in neutral form (His), or plays the cation role in protonated form (His+); (2) π-π stacking interactions between histidine and other aromatic amino acids; (3) Hydrogen-π interactions between histidine and other aromatic amino acids; (4) Coordinate interactions between histidine and metallic cations. The energies of π-π stacking interactions and hydrogen-π interactions are calculated using CCSD/6-31+G(d,p). The energies of cation-π interactions and coordinate interactions are calculated using B3LYP/6-31+G(d,p) method and adjusted by empirical method for dispersion energy.
Conclusions: The coordinate interactions between histidine and metallic cations are the strongest one acting in broad range, followed by the cation-π, hydrogen-π, and π-π stacking interactions. When the histidine is in neutral form, the cation-π interactions are attractive; when it is protonated (His+), the interactions turn to repulsive. The two protonation forms (and pKa values) of histidine are reversibly switched by the attractive and repulsive cation-π interactions. In proteins the π-π stacking interaction between neutral histidine and aromatic amino acids (Phe, Tyr, Trp) are in the range from -3.0 to -4.0 kcal/mol, significantly larger than the van der Waals energies.
Figures
References
-
- Agnieszka M, Janina KW, Katarzyna KK. Five-membered heterocycles. Part III. Aromaticity of 1,3-imidazole in 5+n hetero-bicyclic molecules. J Mol Struc. 2003;655:397–403. doi: 10.1016/S0022-2860(03)00282-5. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

