Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication
- PMID: 23452682
- PMCID: PMC3589108
- DOI: 10.1016/j.jaac.2012.12.014
Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication
Abstract
Objective: To determine long-term effects on substance use and substance use disorder (SUD), up to 8 years after childhood enrollment, of the randomly assigned 14-month treatments in the multisite Multimodal Treatment Study of Children with Attention-Deficit/Hyperactivity Disorder (MTA; n = 436); to test whether medication at follow-up, cumulative psychostimulant treatment over time, or both relate to substance use/SUD; and to compare substance use/SUD in the ADHD sample to the non-ADHD childhood classmate comparison group (n = 261).
Method: Mixed-effects regression models with planned contrasts were used for all tests except the important cumulative stimulant treatment question, for which propensity score matching analysis was used.
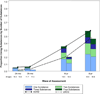
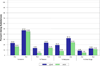
Results: The originally randomized treatment groups did not differ significantly on substance use/SUD by the 8-year follow-up or earlier (mean age = 17 years). Neither medication at follow-up (mostly stimulants) nor cumulative stimulant treatment was associated with adolescent substance use/SUD. Substance use at all time points, including use of two or more substances and SUD, were each greater in the ADHD than in the non-ADHD samples, regardless of sex.
Conclusions: Medication for ADHD did not protect from, or contribute to, visible risk of substance use or SUD by adolescence, whether analyzed as randomized treatment assignment in childhood, as medication at follow-up, or as cumulative stimulant treatment over an 8-year follow-up from childhood. These results suggest the need to identify alternative or adjunctive adolescent-focused approaches to substance abuse prevention and treatment for boys and girls with ADHD, especially given their increased risk for use and abuse of multiple substances that is not improved with stimulant medication. Clinical trial registration information-Multimodal Treatment Study of Children With Attention Deficit and Hyperactivity Disorder (MTA); http://clinical trials.gov/; NCT00000388.
Copyright © 2013 American Academy of Child and Adolescent Psychiatry. All rights reserved.
Conflict of interest statement
Disclosure: Drs. Molina, Hoza, Epstein, Vitiello, Gibbons, Howard, Hur, Lu, and Marcus, and Ms. Houck report no biomedical financial interests or potential conflicts of interest.
Figures
Comment in
-
Do stimulants prevent substance use and misuse among youth with attention-deficit/hyperactivity disorder? The answer is still maybe.J Am Acad Child Adolesc Psychiatry. 2013 Mar;52(3):225-7. doi: 10.1016/j.jaac.2012.12.016. J Am Acad Child Adolesc Psychiatry. 2013. PMID: 23452678 No abstract available.
References
-
- Charach A, Yeung E, Climans T, Lillie E. Childhood Attention-Deficit/Hyperactivity Disorder and future substance use disorders: Comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50(1):9–21. - PubMed
-
- Derefinko KD, Pelham WE. ADHD and substance use. In: Sher KJ, editor. Oxford Handbook of Substance Use Disorders. New York, NY: Oxford University Press; 2013.
-
- Molina BSG. Delinquency and substance use in ADHD: Adolescent and young adult outcomes in developmental context. In: Evans SW, Hoza B, editors. Attention Deficit Hyperactivity Disorder: State of the Science and Best Practices. Vol. 2. Kingston, NJ: Civic Research Institute; 2011.
-
- Pelham WE, Fabiano GA. Evidence-based psychosocial treatment for attention deficit/hyperactivity disorder: An update. J Clin Child Adolesc Psychol. 2008;37(1):185–214. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN271200800005C/DA/NIDA NIH HHS/United States
- U01 MH050467/MH/NIMH NIH HHS/United States
- F32 MH012004/MH/NIMH NIH HHS/United States
- U01MH50453/MH/NIMH NIH HHS/United States
- N01 DA085552/DA/NIDA NIH HHS/United States
- N01 MH012008/MH/NIMH NIH HHS/United States
- DA-8-5550/DA/NIDA NIH HHS/United States
- DA-8-5554/DA/NIDA NIH HHS/United States
- U01 MH050461/MH/NIMH NIH HHS/United States
- DA-8-5549/DA/NIDA NIH HHS/United States
- DA-8-5551/DA/NIDA NIH HHS/United States
- HHSN271200800008C/DA/NIDA NIH HHS/United States
- U01 MH050440/MH/NIMH NIH HHS/United States
- N01 MH012012/MH/NIMH NIH HHS/United States
- HHSN271200800003C/DA/NIDA NIH HHS/United States
- N01 DA085549/DA/NIDA NIH HHS/United States
- N01 MH012010/MH/NIMH NIH HHS/United States
- HHSN271200800009C/DA/NIDA NIH HHS/United States
- HHSN271200800007-C/PHS HHS/United States
- HHSN271200800005-C/PHS HHS/United States
- N01 DA085553/DA/NIDA NIH HHS/United States
- U01MH50467/MH/NIMH NIH HHS/United States
- HHSN271200800006C/DA/NIDA NIH HHS/United States
- HHSN271200800004-C/PHS HHS/United States
- HHSN271200800009-C/PHS HHS/United States
- N01 MH012011/MH/NIMH NIH HHS/United States
- N01 MH012004/MH/NIMH NIH HHS/United States
- HHSN271200800003-C/PHS HHS/United States
- N01 MH012009/MH/NIMH NIH HHS/United States
- U01 MH050453/MH/NIMH NIH HHS/United States
- N01 MH012007/MH/NIMH NIH HHS/United States
- N01 DA085551/DA/NIDA NIH HHS/United States
- HHSN271200800004C/DA/NIDA NIH HHS/United States
- U01MH50477/MH/NIMH NIH HHS/United States
- DA-8-5548/DA/NIDA NIH HHS/United States
- N01 DA085550/DA/NIDA NIH HHS/United States
- HHSN271200800006-C/PHS HHS/United States
- N01 DA085548/DA/NIDA NIH HHS/United States
- DA-8-5553/DA/NIDA NIH HHS/United States
- HHSN271200800008-C/PHS HHS/United States
- U01 MH50461/MH/NIMH NIH HHS/United States
- DA-8-5552/DA/NIDA NIH HHS/United States
- U01MH50440/MH/NIMH NIH HHS/United States
- HHSN271200800007C/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

