Streptococcus endophthalmitis outbreak after intravitreal injection of bevacizumab: one-year outcomes and investigative results
- PMID: 23453511
- PMCID: PMC3702685
- DOI: 10.1016/j.ophtha.2012.12.009
Streptococcus endophthalmitis outbreak after intravitreal injection of bevacizumab: one-year outcomes and investigative results
Abstract
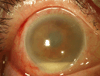
Purpose: To report the 1-year clinical outcomes of an outbreak of Streptococcus endophthalmitis after intravitreal injection of bevacizumab, including visual acuity outcomes, microbiological testing, and compound pharmacy investigations by the Food and Drug Administration (FDA).
Design: Retrospective consecutive case series.
Participants: Twelve eyes of 12 patients who developed endophthalmitis after receiving intravitreal bevacizumab prepared by a single compounding pharmacy.
Methods: Medical records of patients were reviewed; phenotypic and DNA analyses were performed on microbes cultured from patients and from unused syringes. An inspection report by the FDA based on site visits to the pharmacy that prepared the bevacizumab syringes was summarized.
Main outcome measures: Visual acuity, interventions received, time to intervention, microbiological consistency, and FDA inspection findings.
Results: Between July 5 and 8, 2011, 12 patients developed endophthalmitis after intravitreal bevacizumab from syringes prepared by a single compounding pharmacy. All patients received initial vitreous tap and injection, and 8 patients (67%) subsequently underwent pars plana vitrectomy (PPV). After 12 months follow-up, outcomes have been poor. Seven patients (58%) required evisceration or enucleation, and only 1 patient regained pre-injection visual acuity. Molecular testing using real-time polymerase chain reaction, partial sequencing of the groEL gene, and multilocus sequencing of 7 housekeeping genes confirmed the presence of a common strain of Streptococcus mitis/oralis in vitreous specimens and 7 unused syringes prepared by the compounding pharmacy at the same time. An FDA investigation of the compounding pharmacy noted deviations from standard sterile technique, inconsistent documentation, and inadequate testing of equipment required for safe preparation of medications.
Conclusions: In this outbreak of endophthalmitis, outcomes have been generally poor, and PPV did not improve visual results at 1-year follow-up. Molecular testing confirmed a common strain of S. mitis/oralis. Contamination seems to have occurred at the compounding pharmacy, where numerous problems in sterile technique were noted by public health investigators.
Copyright © 2013 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: RAG: Emmetrope Ophthalmics. HWF: Alimera, Santen, Pfizer. DM: none. SG: none. RFI: none. PMB: none. The authors have no financial or proprietary interests in the material presented herein.
Figures







References
-
- McCannel CA. Meta-analysis of endophthalmitis following intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31:654–661. - PubMed
-
- Moshfeghi AA, Rosenfeld PJ, Flynn HW, Jr, et al. Endophthalmitis after intravitreal vascular [corrected] endothelial growth factor antagonists: a six-year experience at a university referral center. Retina. 2011;31:662–668. - PubMed
-
- Wen JC, McCannel CA, Mochon AB, Garner OB. Bacterial dispersal associated with speech in the setting of intravitreous injections. Arch Ophthalmol. 2011;129:1551–1554. - PubMed
-
- Schimel AM, Scott IU, Flynn HW., Jr Endophthalmitis after intravitreal injections: should the use of face masks be the standard of care? Arch Ophthalmol. 2011;129:1607–1609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

