Children with pulmonary arterial hypertension and prostanoid therapy: long-term hemodynamics
- PMID: 23453572
- PMCID: PMC3760159
- DOI: 10.1016/j.healun.2013.01.1055
Children with pulmonary arterial hypertension and prostanoid therapy: long-term hemodynamics
Abstract
Background: Pediatric patients with severe pulmonary arterial hypertension (PAH) are treated with intravenous epoprostenol or intravenous or subcutaneous treprostinil. Little is known about longitudinal hemodynamics and outcomes of epoprostenol, treprostinil, and transitions from epoprostenol to treprostinil.
Methods: This was retrospective study of 77 pediatric patients (47 idiopathic PAH, 24 congenital heart disease-PAH) receiving epoprostenol or treprostinil from 1992 to 2010 at 2 centers. Outcomes were defined as living vs dead/transplant.
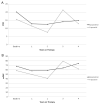
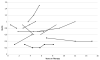
Results: Mean age at baseline was 7.7 ± 5.2 years, with follow-up of 4.3 ± 3.4 years. Thirty-seven patients were treated with epoprostenol, 20 with treprostinil, and 20 were transitioned from epoprostenol to treprostinil. Mean pulmonary-to-systemic vascular resistance ratio (Rp/Rs) for epoprostenol was 1.0 ± 0.4, 0.8 ± 0.4, 0.8 ± 0.4, 1.0 ± 0.4, and 1.2 ± 0.4, respectively, at baseline, 1, 2, 3, and 4 years. For treprostinil, Rp/Rs was 0.9 ± 0.3, 0.7 ± 0.3, 0.5 ± 0.2, (p < 0.01 vs baseline), and 1.1 ± 0.2, respectively, at baseline, 1, 2, and 3 to 4 years, respectively. There were similar changes in mean pulmonary artery pressure and pulmonary vascular resistance index. The Rp/Rs 1 year after epoprostenol to treprostinil transition increased from 0.6 to 0.8 (n = 7). Changes not statistically significant unless noted. Eight patients died or received a transplant within 2 years of baseline; compared with the rest of the cohort, mean baseline Rp/Rs, right atrial pressure, and pulmonary vascular resistance index were significantly worse in this group. Thirty-nine patients remain on prostanoids, 17 are off, 16 died, and 5 received heart-lung transplant. Kaplan-Meier 5-year transplant-free survival was 70% (95% confidence interval, 56%-80%).
Conclusion: There was improvement in Rp/Rs on both therapies at 1 to 2 years that was not sustained. The 5-year transplant-free survival was better than in similar adult studies.
Copyright © 2013 International Society for Heart and Lung Transplantation. Published by Elsevier Inc. All rights reserved.
Figures
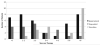



References
-
- D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. - PubMed
-
- Rosenzweig EB, Widlitz AC, Barst RJ. Pulmonary arterial hypertension in children. Pediatr Pulmonol. 2004;38:2–22. - PubMed
-
- Humpl T, Reyes JT, Holtby H, Stephens D, Adatia I. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005;111:3274–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

