Genetic basis of cell-cell fusion mechanisms
- PMID: 23453622
- PMCID: PMC4022042
- DOI: 10.1016/j.tig.2013.01.011
Genetic basis of cell-cell fusion mechanisms
Abstract
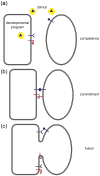
Cell-cell fusion in sexually reproducing organisms is a mechanism to merge gamete genomes and, in multicellular organisms, it is a strategy to sculpt organs, such as muscle, bone, and placenta. Moreover, this mechanism has been implicated in pathological conditions, such as infection and cancer. Studies of genetic model organisms have uncovered a unifying principle: cell fusion is a genetically programmed process. This process can be divided in three stages: competence (cell induction and differentiation); commitment (cell determination, migration, and adhesion); and cell fusion (membrane merging and cytoplasmic mixing). Recent work has led to the discovery of fusogens, which are cell fusion proteins that are necessary and sufficient to fuse cell membranes. Two unrelated families of fusogens have been discovered, one in mouse placenta and one in Caenorhabditis elegans (syncytins and F proteins, respectively). Current research aims to identify new fusogens and determine the mechanisms by which they merge membranes.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




References
-
- Avinoam O, Podbilewicz B. Eukaryotic cell-cell fusion families. Curr Top Membr. 2011;68:209–234. - PubMed
-
- Vaney MC, Rey FA. Class II enveloped viruses. Cell Microbiol. 2011;13:1451–1459. - PubMed
-
- Backovic M, Jardetzky TS. Class III viral membrane fusion proteins. Advances in experimental medicine and biology. 2011;714:91–101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

