AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1
- PMID: 23453806
- PMCID: PMC3615143
- DOI: 10.1016/j.molcel.2013.01.035
AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1
Abstract
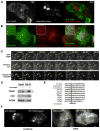
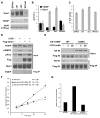
Thioredoxin-interacting protein (TXNIP) is an α-arrestin family protein that is induced in response to glucose elevation. It has been shown to provide a negative feedback loop to regulate glucose uptake into cells, though the biochemical mechanism of action has been obscure. Here, we report that TXNIP suppresses glucose uptake directly, by binding to the glucose transporter GLUT1 and inducing GLUT1 internalization through clathrin-coated pits, as well as indirectly, by reducing the level of GLUT1 messenger RNA (mRNA). In addition, we show that energy stress results in the phosphorylation of TXNIP by AMP-dependent protein kinase (AMPK), leading to its rapid degradation. This suppression of TXNIP results in an acute increase in GLUT1 function and an increase in GLUT1 mRNA (hence the total protein levels) for long-term adaptation. The glucose influx through GLUT1 restores ATP-to-ADP ratios in the short run and ultimately induces TXNIP protein production to suppress glucose uptake once energy homeostasis is reestablished.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Augustin R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB life. 2010;62:315–333. - PubMed
-
- Baker AF, Koh MY, Williams RR, James B, Wang H, Tate WR, Gallegos A, Von Hoff DD, Han H, Powis G. Identification of thioredoxin-interacting protein 1 as a hypoxia-inducible factor 1alpha-induced gene in pancreatic cancer. Pancreas. 2008;36:178–186. - PubMed
-
- Billiet L, Furman C, Cuaz-Perolin C, Paumelle R, Raymondjean M, Simmet T, Rouis M. Thioredoxin-1 and its natural inhibitor, vitamin D3 up-regulated protein 1, are differentially regulated by PPARalpha in human macrophages. Journal of molecular biology. 2008;384:564–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5P30CA006516/CA/NCI NIH HHS/United States
- R37 DK43051/DK/NIDDK NIH HHS/United States
- 5P01CA120964/CA/NCI NIH HHS/United States
- R01 CA166717/CA/NCI NIH HHS/United States
- NIH R01-GM56302/GM/NIGMS NIH HHS/United States
- R56 DK052852/DK/NIDDK NIH HHS/United States
- R00-CA133245/CA/NCI NIH HHS/United States
- R37 DK043051/DK/NIDDK NIH HHS/United States
- R01 DK043051/DK/NIDDK NIH HHS/United States
- R01 GM056203/GM/NIGMS NIH HHS/United States
- P01 CA120964/CA/NCI NIH HHS/United States
- R01 DK052852/DK/NIDDK NIH HHS/United States
- DK52852/DK/NIDDK NIH HHS/United States
- P01-CA120964/CA/NCI NIH HHS/United States
- P30 CA006516/CA/NCI NIH HHS/United States
- R01 DK098002/DK/NIDDK NIH HHS/United States
- R00 CA133245/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

