Rheotaxis guides mammalian sperm
- PMID: 23453951
- PMCID: PMC3607503
- DOI: 10.1016/j.cub.2013.02.007
Rheotaxis guides mammalian sperm
Abstract
Background: In sea urchins, spermatozoan motility is altered by chemotactic peptides, giving rise to the assumption that mammalian eggs also emit chemotactic agents that guide spermatozoa through the female reproductive tract to the mature oocyte. Mammalian spermatozoa indeed undergo complex adaptations within the female (the process of capacitation) that are initiated by agents ranging from pH to progesterone, but these factors are not necessarily taxic. Currently, chemotaxis, thermotaxis, and rheotaxis have not been definitively established in mammals.
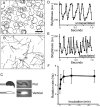
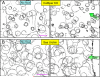
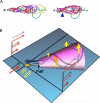
Results: Here, we show that positive rheotaxis, the ability of organisms to orient and swim against the flow of surrounding fluid, is a major taxic factor for mouse and human sperm. This flow is generated within 4 hr of sexual stimulation and coitus in female mice; prolactin-triggered oviductal fluid secretion clears the oviduct of debris, lowers viscosity, and generates the stream that guides sperm migration in the oviduct. Rheotaxic movement is demonstrated in capacitated and uncapacitated spermatozoa in low- and high-viscosity media. Finally, we show that a unique sperm motion, which we quantify using the sperm head's rolling rate, reflects sperm rotation that generates essential force for positioning the sperm in the stream. Rotation requires CatSper channels, presumably by enabling Ca(2+) influx.
Conclusions: We propose that rheotaxis is a major determinant of sperm guidance over long distances in the mammalian female reproductive tract. Coitus induces fluid flow to guide sperm in the oviduct. Sperm rheotaxis requires rotational motion during CatSper channel-dependent hyperactivated motility.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




References
-
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm--making the most of what you've got. Nat Cell Biol. 2007;9:235–242. - PubMed
-
- Eisenbach M, Giojalas LC. Sperm guidance in mammals - an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–285. - PubMed
-
- Fukuda N, Yomogida K, Okabe M, Touhara K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J Cell Sci. 2004;117:5835–5845. - PubMed
-
- Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. - PubMed
-
- Eisenbach M. Mammalian sperm chemotaxis and its association with capacitation. Dev Genet. 1999;25:87–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

