Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury
- PMID: 23454065
- PMCID: PMC3680365
- DOI: 10.1016/j.freeradbiomed.2013.02.018
Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury
Abstract
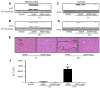
Acetaminophen (APAP), a widely used analgesic/antipyretic agent, can cause liver injury through increased nitrative stress, leading to protein nitration. However, the identities of nitrated proteins and their roles in hepatotoxicity are poorly understood. Thus, we aimed at studying the mechanism of APAP-induced hepatotoxicity by systematic identification and characterization of nitrated proteins in the absence or presence of an antioxidant, N-acetylcysteine (NAC). The levels of nitrated proteins markedly increased at 2h in mice exposed to a single APAP dose (350mg/kg ip), which caused severe liver necrosis at 24h. Protein nitration and liver necrosis were minimal in mice exposed to nontoxic 3-hydroxyacetanilide or animals co-treated with APAP and NAC. Mass-spectral analysis of the affinity-purified nitrated proteins identified numerous mitochondrial and cytosolic proteins, including mitochondrial aldehyde dehydrogenase, Mn-superoxide dismutase, glutathione peroxidase, ATP synthase, and 3-ketoacyl-CoA thiolase, involved in antioxidant defense, energy supply, or fatty acid metabolism. Immunoprecipitation followed by immunoblot with anti-3-nitrotyrosine antibody confirmed that the aforementioned proteins were nitrated in APAP-exposed mice but not in NAC-cotreated mice. Consistently, NAC cotreatment significantly restored the suppressed activity of these enzymes. Thus, we demonstrate a new mechanism by which many nitrated proteins with concomitantly suppressed activity promotes APAP-induced mitochondrial dysfunction and hepatotoxicity.
Published by Elsevier Inc.
Conflict of interest statement
AUTHOR DISCLOSURE STATEMENT
The authors declare that they do not have any competing financial interests.
Figures





References
-
- Bae MA, Song BJ. Critical role of c-Jun N-terminal protein kinase activation in troglitazone-induced apoptosis of human HepG2 hepatoma cells. Mol Pharmacol. 2003;63:401–408. - PubMed
-
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. - PubMed
-
- Carmiel-Haggai M, Cederbaum AI, Nieto N. Binge ethanol exposure increases liver injury in obese rats. Gastroenterology. 2003;125:1818–1833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

