Comparative effectiveness of the nicotine lozenge and tobacco-free snuff for smokeless tobacco reduction
- PMID: 23454876
- PMCID: PMC3595368
- DOI: 10.1016/j.addbeh.2013.01.023
Comparative effectiveness of the nicotine lozenge and tobacco-free snuff for smokeless tobacco reduction
Abstract
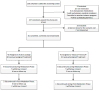
Long-term smokeless tobacco (ST) use is associated with cardiovascular disease and cancer, but not all ST users want to quit. Previous studies have evaluated the effectiveness of nicotine lozenges and tobacco-free snuff for reducing ST use among ST users not ready to quit, but no comparative effectiveness trials of these two products have been conducted. We conducted a multicenter, randomized clinical pilot study evaluating the comparative effectiveness of the 4-mg nicotine lozenge and tobacco-free snuff for reducing ST use and increasing tobacco abstinence among ST users with no intention of quitting in the next 30 days. Participants received 8 weeks of treatment and behavioral counseling on tobacco reduction strategies with follow-up to 26 weeks. We randomized 81 participants (40 nicotine lozenges, 41 tobacco-free snuff). No significant differences in reduction were observed between the two groups at weeks 8, 12, and 26. No significant differences were observed between groups in nicotine withdrawal or tobacco craving. However, both groups significantly reduced (p<.001) ST use in cans/week and dips/day from baseline which was sustained through the end-of-study. The observed biochemically-confirmed abstinence rates at week 26 were similar between groups (12% vs. 12%, one-tailed p=.615). The 4-mg nicotine lozenge and the tobacco-free snuff both appear to be effective and comparable for reducing ST use among ST users not ready to quit in the next 30 days.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Dr. Jon O. Ebbert has received consulting fees from GlaxoSmithKline, manufacturer of the nicotine lozenge.
Figures
References
-
- Akers L, Severson HH, Yovanoff P, Boles SM. Oregon Research Institute Tobacco Workgroup Technical Report. 2011. Eugene, OR: Oregon Research Institute; 2011. Using Item Response Theory to Develop Models of Smokeless Tobacco Dependence.
-
- Batra A, Klingler K, Landfeldt B, Friederich HM, Westin A, Danielsson T. Smoking reduction treatment with 4-mg nicotine gum: a double-blind, randomized, placebo-controlled study. Clinical Pharmacology and Therapeutics. 2005;78:689–696. - PubMed
-
- Benowitz NL, Ahijevych K, Hall S, Hansson A, Henningfield J, Hurt RD, Jacob PI, Jarvis MJ, LeHouezec J, Lichtenstein E, Tsoh J, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. - PubMed
-
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology. 2004;72:371–381. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical