NKG2D blockade inhibits poly(I:C)-triggered fetal loss in wild type but not in IL-10-/- mice
- PMID: 23455498
- PMCID: PMC3608719
- DOI: 10.4049/jimmunol.1203488
NKG2D blockade inhibits poly(I:C)-triggered fetal loss in wild type but not in IL-10-/- mice
Abstract
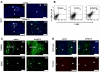
Infection and inflammation can disturb immune tolerance at the maternal-fetal interface, resulting in adverse pregnancy outcomes. However, the underlying mechanisms for detrimental immune responses remain ill defined. In this study, we provide evidence for immune programming of fetal loss in response to polyinosinic:polycytidylic acid (polyI:C), a viral mimic and an inducer of inflammatory milieu. IL-10 and uterine NK (uNK) cells expressing the activating receptor NKG2D play a critical role in poly(I:C)-induced fetal demise. In wild type (WT) mice, poly(I:C) treatment induced expansion of NKG2D(+) uNK cells and expression of Rae-1 (an NKG2D ligand) on uterine macrophages and led to fetal resorption. In IL-10(-/-) mice, NKG2D(-) T cells instead became the source of fetal resorption during the same gestation period. Interestingly, both uterine NK and T cells produced TNF-α as the key cytotoxic factor contributing to fetal loss. Treatment of WT mice with poly(I:C) resulted in excessive trophoblast migration into the decidua and increased TUNEL-positive signal. IL-10(-/-) mice supplemented with recombinant IL-10 induced fetal loss through NKG2D(+) uNK cells, similar to the response in WT mice. Blockade of NKG2D in poly(I:C)-treated WT mice led to normal pregnancy outcome. Thus, we demonstrate that pregnancy-disrupting inflammatory events mimicked by poly(I:C) are regulated by IL-10 and depend on the effector function of uterine NKG2D(+) NK cells in WT mice and NKG2D(-) T cells in IL-10 null mice.
Figures



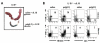


Similar articles
-
NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis.Hepatology. 2009 Mar;49(3):940-9. doi: 10.1002/hep.22725. Hepatology. 2009. PMID: 19177594
-
Activating receptor NKG2D targets RAE-1-expressing allogeneic neural precursor cells in a viral model of multiple sclerosis.Stem Cells. 2014 Oct;32(10):2690-701. doi: 10.1002/stem.1760. Stem Cells. 2014. PMID: 24898518 Free PMC article.
-
Kupffer Cells Regulate Natural Killer Cells Via the NK group 2, Member D (NKG2D)/Retinoic Acid Early Inducible-1 (RAE-1) Interaction and Cytokines in a Primary Biliary Cholangitis Mouse Model.Med Sci Monit. 2020 Jun 29;26:e923726. doi: 10.12659/MSM.923726. Med Sci Monit. 2020. PMID: 32599603 Free PMC article.
-
Evolution of non-cytotoxic uterine natural killer cells.Am J Reprod Immunol. 2008 May;59(5):425-32. doi: 10.1111/j.1600-0897.2008.00595.x. Am J Reprod Immunol. 2008. PMID: 18405313 Free PMC article. Review.
-
Interleukin-15 in Outcomes of Pregnancy.Int J Mol Sci. 2021 Oct 14;22(20):11094. doi: 10.3390/ijms222011094. Int J Mol Sci. 2021. PMID: 34681751 Free PMC article. Review.
Cited by
-
Potential effects of SARS-CoV-2 infection during pregnancy on fetuses and newborns are worthy of attention.J Obstet Gynaecol Res. 2020 Oct;46(10):1951-1957. doi: 10.1111/jog.14406. Epub 2020 Aug 10. J Obstet Gynaecol Res. 2020. PMID: 32779309 Free PMC article. Review.
-
Toxoplasma gondii infection of decidual CD1c(+) dendritic cells enhances cytotoxicity of decidual natural killer cells.Inflammation. 2014 Aug;37(4):1261-70. doi: 10.1007/s10753-014-9853-x. Inflammation. 2014. PMID: 24573986
-
Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism.Transl Psychiatry. 2019 Mar 28;9(1):124. doi: 10.1038/s41398-019-0457-y. Transl Psychiatry. 2019. PMID: 30923308 Free PMC article.
-
Endogenous TWEAK is critical for regulating the function of mouse uterine natural killer cells in an immunological model of pregnancy loss.Immunology. 2016 May;148(1):70-82. doi: 10.1111/imm.12588. Epub 2016 Mar 2. Immunology. 2016. PMID: 27040357 Free PMC article.
-
Interferon response factor 3 is crucial to poly-I:C induced NK cell activity and control of B16 melanoma growth.Cancer Lett. 2014 Apr 28;346(1):122-8. doi: 10.1016/j.canlet.2013.12.022. Epub 2013 Dec 22. Cancer Lett. 2014. PMID: 24368188 Free PMC article.
References
-
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000;342:1500–1507. - PubMed
-
- Haun L, Kwan N, Hollier LM. Viral infections in pregnancy. Minerva. Ginecol. 2007;59:159–174. - PubMed
-
- Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin. Neonatol. 2002;7:259–274. - PubMed
-
- Moffet A, Loke C. Immunology of placentation in eutharian mammals. Nat. Rev. Immunol. 2006;8:584–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

