Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor
- PMID: 23455672
- PMCID: PMC6269682
- DOI: 10.3390/molecules18032821
Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor
Abstract
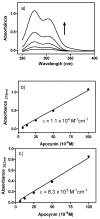
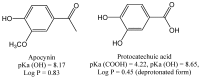
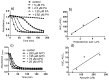
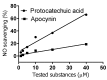
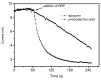
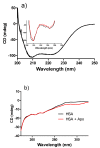
Apocynin is the most employed inhibitor of NADPH oxidase (NOX), a multienzymatic complex capable of catalyzing the one-electron reduction of molecular oxygen to the superoxide anion. Despite controversies about its selectivity, apocynin has been used as one of the most promising drugs in experimental models of inflammatory and neurodegenerative diseases. Here, we aimed to study the chemical and biophysical properties of apocynin. The oxidation potential was determined by cyclic voltammetry (Epa = 0.76V), the hydrophobicity index was calculated (logP = 0.83) and the molar absorption coefficient was determined (e275nm = 1.1 × 104 M-1 cm-1). Apocynin was a weak free radical scavenger (as measured using the DPPH, peroxyl radical and nitric oxide assays) when compared to protocatechuic acid, used here as a reference antioxidant. On the other hand, apocynin was more effective than protocatechuic acid as scavenger of the non-radical species hypochlorous acid. Apocynin reacted promptly with the non-radical reactive species H2O2 only in the presence of peroxidase. This finding is relevant, since it represents a new pathway for depleting H2O2 in cellular experimental models, besides the direct inhibition of NADPH oxidase. This could be relevant for its application as an inhibitor of NOX4, since this isoform produces H2O2 and not superoxide anion. The binding parameters calculated by fluorescence quenching showed that apocynin binds to human serum albumin (HSA) with a binding affinity of 2.19 × 104 M-1. The association did not alter the secondary and tertiary structure of HSA, as verified by synchronous fluorescence and circular dichroism. The displacement of fluorescent probes suggested that apocynin binds to site I and site II of HSA. Considering the current biomedical applications of this phytochemical, the dissemination of these chemical and biophysical properties can be very helpful for scientists and physicians interested in the use of apocynin.
Figures





Similar articles
-
Protocatechuic acid alkyl esters: hydrophobicity as a determinant factor for inhibition of NADPH oxidase.Curr Med Chem. 2012;19(28):4885-93. doi: 10.2174/092986712803341557. Curr Med Chem. 2012. PMID: 22934778
-
Ethers and esters derived from apocynin avoid the interaction between p47phox and p22phox subunits of NADPH oxidase: evaluation in vitro and in silico.Biosci Rep. 2013 Aug 2;33(4):e00055. doi: 10.1042/BSR20130029. Biosci Rep. 2013. PMID: 23802190 Free PMC article.
-
Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant.Hypertension. 2008 Feb;51(2):211-7. doi: 10.1161/HYPERTENSIONAHA.107.100214. Epub 2007 Dec 17. Hypertension. 2008. PMID: 18086956
-
The role of the methoxyphenol apocynin, a vascular NADPH oxidase inhibitor, as a chemopreventative agent in the potential treatment of cardiovascular diseases.Curr Vasc Pharmacol. 2008 Jul;6(3):204-17. doi: 10.2174/157016108784911984. Curr Vasc Pharmacol. 2008. PMID: 18673160 Review.
-
Apocynin: molecular aptitudes.Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. Epub 2008 Dec 2. Mediators Inflamm. 2008. PMID: 19096513 Free PMC article. Review.
Cited by
-
Methyl divanillate: redox properties and binding affinity with albumin of an antioxidant and potential NADPH oxidase inhibitor.RSC Adv. 2019 Jun 26;9(35):19983-19992. doi: 10.1039/c9ra02465d. eCollection 2019 Jun 25. RSC Adv. 2019. PMID: 35514705 Free PMC article.
-
Carbohydrate microarrays for screening functional glycans.Chem Sci. 2016 Mar 1;7(3):2084-2093. doi: 10.1039/c5sc03789a. Epub 2016 Jan 5. Chem Sci. 2016. PMID: 29899934 Free PMC article.
-
Propofol Protects Against H2O2-Induced Oxidative Injury in Differentiated PC12 Cells via Inhibition of Ca(2+)-Dependent NADPH Oxidase.Cell Mol Neurobiol. 2016 May;36(4):541-51. doi: 10.1007/s10571-015-0235-1. Epub 2015 Jul 11. Cell Mol Neurobiol. 2016. PMID: 26162968 Free PMC article.
-
Comparative study between apocynin and protocatechuic acid regarding antioxidant capacity and vascular effects.Front Physiol. 2022 Nov 15;13:1047916. doi: 10.3389/fphys.2022.1047916. eCollection 2022. Front Physiol. 2022. PMID: 36457305 Free PMC article.
-
Antioxidants protect against diabetes by improving glucose homeostasis in mouse models of inducible insulin resistance and obesity.Diabetologia. 2019 Nov;62(11):2094-2105. doi: 10.1007/s00125-019-4937-7. Epub 2019 Jul 15. Diabetologia. 2019. PMID: 31309261 Free PMC article.
References
-
- Kleniewska P., Piechota A., Skibska B., Gorąca A. The NADPH oxidase family and its inhibitors. Arch. Immunol. Ther. Exp. (Warsz) 2012;60:277–294. - PubMed
-
- Stolk J., Hiltermann T.J., Dijkman J.H., Verhoeven A.J. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 1994;11:95–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

