Modern concepts concerning the origin of the heartbeat
- PMID: 23455768
- PMCID: PMC3768086
- DOI: 10.1152/physiol.00054.2012
Modern concepts concerning the origin of the heartbeat
Abstract
Physiological processes governing the heart beat have been under investigation for several hundred years. Major advances have been made in the recent past. A review of the present paradigm is presented here, including a look back at important steps that led us to where we are today, alongside a glimpse into the exciting future of pacemaker research.
Figures


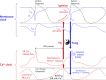
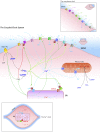
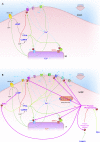





References
-
- Bakker ML, Boink GJ, Boukens BJ, Verkerk AO, van den Boogaard M, den Haan AD, et al. T-box transcription factor TBX3 reprogrammes mature cardiac myocytes into pacemaker-like cells. Cardiovasc Res 94: 439–449, 2012. - PubMed
-
- Baruscotti M, Bottelli G, Milanesi R, DiFrancesco JC, DiFrancesco D. HCN-related channelopathies. Pflügers Arch 460: 405–415, 2010. - PubMed
-
- Baruscotti M, Bucchi A, DiFrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther 107: 59–79, 2005. - PubMed
-
- Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na+-Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res 88: 1254–1258, 2001. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

