Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration?
- PMID: 23456256
- PMCID: PMC11113366
- DOI: 10.1007/s00018-013-1290-8
Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration?
Abstract
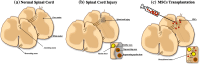
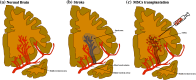
The low regeneration potential of the central nervous system (CNS) represents a challenge for the development of new therapeutic strategies. Mesenchymal stem cells (MSCs) have been proposed as a possible therapeutic tool for CNS disorders. In addition to their differentiation potential, it is well accepted nowadays that their beneficial actions can also be mediated by their secretome. Indeed, it was already demonstrated, both in vitro and in vivo, that MSCs are able to secrete a broad range of neuroregulatory factors that promote an increase in neurogenesis, inhibition of apoptosis and glial scar formation, immunomodulation, angiogenesis, neuronal and glial cell survival, as well as relevant neuroprotective actions on different pathophysiological contexts. Considering their protective action in lesioned sites, MSCs' secretome might also improve the integration of local progenitor cells in neuroregeneration processes, opening a door for their future use as therapeutical strategies in human clinical trials. Thus, in this review we analyze the current understanding of MSCs secretome as a new paradigm for the treatment of CNS neurodegenerative diseases.
Figures


References
-
- Gogel S, Gubernator M, Minger SL. Progress and prospects: stem cells and neurological diseases. Gene Ther. 2011;18(1):1–6. - PubMed
-
- Becerra J, Santos-Ruiz L, Andrades JA, et al. The stem cell niche should be a key issue for cell therapy in regenerative medicine. Stem Cell Rev. 2011;7(2):248–255. - PubMed
-
- Chen FM, Wu LA, Zhang M, et al. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32(12):3189–3209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

