Motor and nonmotor complications in Parkinson's disease: an argument for continuous drug delivery?
- PMID: 23456290
- PMCID: PMC3751411
- DOI: 10.1007/s00702-013-0981-5
Motor and nonmotor complications in Parkinson's disease: an argument for continuous drug delivery?
Abstract
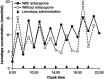
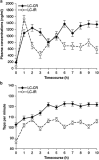
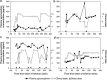
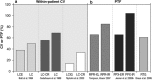
The complications of long-term levodopa therapy for Parkinson's disease (PD) include motor fluctuations, dyskinesias, and also nonmotor fluctuations-at least equally common, but less well appreciated-in autonomic, cognitive/psychiatric, and sensory symptoms. In seeking the pathophysiologic mechanisms, the leading hypothesis is that in the parkinsonian brain, intermittent, nonphysiological stimulation of striatal dopamine receptors destabilizes an already unstable system. Accordingly, a major goal of PD treatment in recent years has been the attainment of continuous dopaminergic stimulation (CDS)-or, less theoretically (and more clinically verifiable), continuous drug delivery (CDD). Improvements in the steadiness of the plasma profiles of various dopaminergic therapies may be a signal of progress. However, improvements in plasma profile do not necessarily translate into CDS, or even into CDD to the brain. Still, it is reassuring that clinical studies of approaches to CDD have generally been positive. Head-to-head comparative trials have often failed to uncover evidence favoring such approaches over an intermittent therapy. Nevertheless, the findings among recipients of subcutaneous apomorphine infusion or intrajejunal levodopa/carbidopa intestinal gel suggest that nonmotor PD symptoms or complications may improve in tandem with motor improvement. In vivo receptor binding studies may help to determine the degree of CDS that a dopaminergic therapy can confer. This may be a necessary first step toward establishing whether CDS is, in fact, an important determinant of clinical efficacy. Certainly, the complexities of optimal PD management, and the rationale for an underlying strategy such as CDS or CDD, have not yet been thoroughly elucidated.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

