Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala
- PMID: 23457122
- PMCID: PMC3627558
- DOI: 10.1093/toxsci/kft035
Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala
Abstract
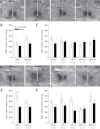
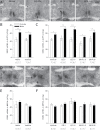
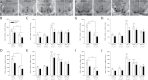
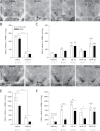
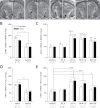
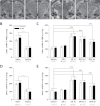
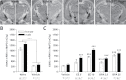
Bisphenol A (BPA) exposure is ubiquitous, and in laboratory animals, early-life BPA exposure has been shown to alter sex-specific neural organization, neuroendocrine physiology, and behavior. The specific mechanisms underlying these brain-related outcomes, however, remain largely unknown, constraining the capacity to ascertain the potential human relevance of neural effects observed in animal models. In the perinatal rat brain, estrogen is masculinizing, suggesting that BPA-induced perturbation of estrogen receptor (ESR) expression may underpin later in-life neuroendocrine effects. We hypothesized that prenatal BPA exposure alters sex-specific ESR1 (ERα) and ESR2 (ERβ) expression in postnatal limbic nuclei. Sprague Dawley rats were mated and gavaged on gestational days (GDs) 6-21 with vehicle, 2.5 or 25 μg/kg bw/day BPA, or 5 or 10 μg/kg bw/day ethinyl estradiol. An additional group was restrained but not gavaged (naïve control). Offspring were sacrificed the day after birth to quantify ESR gene expression throughout the hypothalamus and amygdala by in situ hybridization. Relative to the vehicle group, significant effects of BPA were observed on ESR1 and ESR2 expression throughout the mediobasal hypothalamus and amygdala in both sexes. Significant differences in ESR expression were also observed in the mediobasal hypothalamus and amygdala of the naïve control group compared with the vehicle group, highlighting the potential for gavage to influence gene expression in the developing brain. These results indicate that ESR expression in the neonatal brain of both sexes can be altered by low-dose prenatal BPA exposure.
Figures
References
-
- Aksglaede L., Juul A., Leffers H., Skakkebaek N. E., Andersson A. M. (2006). The sensitivity of the child to sex steroids: Possible impact of exogenous estrogens. Hum. Reprod. Update. 12, 341–349 - PubMed
-
- Ashby J., Lefevre P. A. (1997). The weanling male rat as an assay for endocrine disruption: Preliminary observations. Regul. Toxicol. Pharmacol. 26, 330–337 - PubMed
-
- Bai Y., Chang F., Zhou R., Jin P. P., Matsumoto H., Sokabe M., Chen L. (2011). Increase of anteroventral periventricular kisspeptin neurons and generation of E2-induced LH-surge system in male rats exposed perinatally to environmental dose of bisphenol-A. Endocrinology 152, 1562–1571 - PubMed
-
- Balcombe J. P., Barnard N. D., Sandusky C. (2004). Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 43, 42–51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

