Signaling and transcriptional networks in heart development and regeneration
- PMID: 23457256
- PMCID: PMC3578359
- DOI: 10.1101/cshperspect.a008292
Signaling and transcriptional networks in heart development and regeneration
Abstract
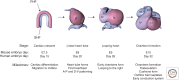
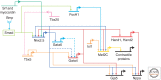
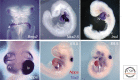
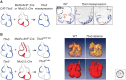
The mammalian heart is the first functional organ, the first indicator of life. Its normal formation and function are essential for fetal life. Defects in heart formation lead to congenital heart defects, underscoring the finesse with which the heart is assembled. Understanding the regulatory networks controlling heart development have led to significant insights into its lineage origins and morphogenesis and illuminated important aspects of mammalian embryology, while providing insights into human congenital heart disease. The mammalian heart has very little regenerative potential, and thus, any damage to the heart is life threatening and permanent. Knowledge of the developing heart is important for effective strategies of cardiac regeneration, providing new hope for future treatments for heart disease. Although we still have an incomplete picture of the mechanisms controlling development of the mammalian heart, our current knowledge has important implications for embryology and better understanding of human heart disease.
Figures

References
-
- Aanhaanen WT, Brons JF, Dominguez JN, Rana MS, Norden J, Airik R, Wakker V, de Gier-de Vries C, Brown NA, Kispert A, et al. 2009. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ Res 104: 1267–1274 - PubMed
-
- Bajolle F, Zaffran S, Kelly RG, Hadchouel J, Bonnet D, Brown NA, Buckingham ME 2006. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ Res 98: 421–428 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
