Loss of ALS-associated TDP-43 in zebrafish causes muscle degeneration, vascular dysfunction, and reduced motor neuron axon outgrowth
- PMID: 23457265
- PMCID: PMC3612625
- DOI: 10.1073/pnas.1218311110
Loss of ALS-associated TDP-43 in zebrafish causes muscle degeneration, vascular dysfunction, and reduced motor neuron axon outgrowth
Abstract
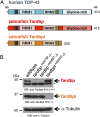
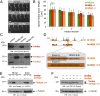
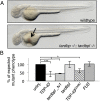
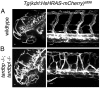
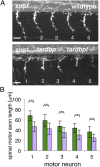
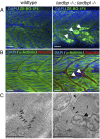
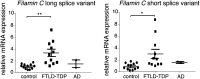
Mutations in the Tar DNA binding protein of 43 kDa (TDP-43; TARDBP) are associated with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with TDP-43(+) inclusions (FTLD-TDP). To determine the physiological function of TDP-43, we knocked out zebrafish Tardbp and its paralogue Tardbp (TAR DNA binding protein-like), which lacks the glycine-rich domain where ALS- and FTLD-TDP-associated mutations cluster. tardbp mutants show no phenotype, a result of compensation by a unique splice variant of tardbpl that additionally contains a C-terminal elongation highly homologous to the glycine-rich domain of tardbp. Double-homozygous mutants of tardbp and tardbpl show muscle degeneration, strongly reduced blood circulation, mispatterning of vessels, impaired spinal motor neuron axon outgrowth, and early death. In double mutants the muscle-specific actin binding protein Filamin Ca is up-regulated. Strikingly, Filamin C is similarly increased in the frontal cortex of FTLD-TDP patients, suggesting aberrant expression in smooth muscle cells and TDP-43 loss-of-function as one underlying disease mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Tardbpl splicing rescues motor neuron and axonal development in a mutant tardbp zebrafish.Hum Mol Genet. 2013 Jun 15;22(12):2376-86. doi: 10.1093/hmg/ddt082. Epub 2013 Feb 19. Hum Mol Genet. 2013. PMID: 23427147 Free PMC article.
-
Neuromuscular junction abnormalities in a zebrafish loss-of-function model of TDP-43.J Neurophysiol. 2019 Jan 1;121(1):285-297. doi: 10.1152/jn.00265.2018. Epub 2018 Nov 21. J Neurophysiol. 2019. PMID: 30461368
-
Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis.Hum Mol Genet. 2009 Oct 15;18(R2):R156-62. doi: 10.1093/hmg/ddp303. Hum Mol Genet. 2009. PMID: 19808791 Free PMC article. Review.
-
Mutant TDP-43 Causes Early-Stage Dose-Dependent Motor Neuron Degeneration in a TARDBP Knockin Mouse Model of ALS.Cell Rep. 2019 Jan 8;26(2):364-373.e4. doi: 10.1016/j.celrep.2018.12.045. Cell Rep. 2019. PMID: 30625319
-
[Optogenetic interrogation of TDP-43 cytotoxicity in a zebrafish ALS model].Nihon Yakurigaku Zasshi. 2023;158(1):16-20. doi: 10.1254/fpj.22085. Nihon Yakurigaku Zasshi. 2023. PMID: 36596480 Review. Japanese.
Cited by
-
RNA-processing protein TDP-43 regulates FOXO-dependent protein quality control in stress response.PLoS Genet. 2014 Oct 16;10(10):e1004693. doi: 10.1371/journal.pgen.1004693. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25329970 Free PMC article.
-
Tiny giants of gene regulation: experimental strategies for microRNA functional studies.Wiley Interdiscip Rev Dev Biol. 2016 May-Jun;5(3):311-62. doi: 10.1002/wdev.223. Epub 2016 Mar 7. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 26950183 Free PMC article. Review.
-
Amyotrophic lateral sclerosis mutant TDP-43 may cause synaptic dysfunction through altered dendritic spine function.Dis Model Mech. 2019 May 17;12(5):dmm038109. doi: 10.1242/dmm.038109. Dis Model Mech. 2019. PMID: 31036551 Free PMC article.
-
Between new genetic discoveries and large randomized trials--neurological research in the era of systems medicine.EMBO Rep. 2013 Jun;14(6):489-92. doi: 10.1038/embor.2013.70. Epub 2013 May 14. EMBO Rep. 2013. PMID: 23670197 Free PMC article.
-
TDP-43 dysregulation and neuromuscular junction disruption in amyotrophic lateral sclerosis.Transl Neurodegener. 2022 Dec 27;11(1):56. doi: 10.1186/s40035-022-00331-z. Transl Neurodegener. 2022. PMID: 36575535 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

