Erythropoietin
- PMID: 23457296
- PMCID: PMC3579209
- DOI: 10.1101/cshperspect.a011619
Erythropoietin
Abstract
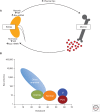
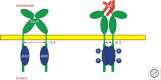
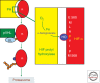
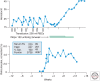
During the past century, few proteins have matched erythropoietin (Epo) in capturing the imagination of physiologists, molecular biologists, and, more recently, physicians and patients. Its appeal rests on its commanding role as the premier erythroid cytokine, the elegant mechanism underlying the regulation of its gene, and its remarkable impact as a therapeutic agent, arguably the most successful drug spawned by the revolution in recombinant DNA technology. This concise review will begin with a synopsis of the colorful history of this protein, culminating in its purification and molecular cloning. It then covers in more detail the contemporary understanding of Epo's physiology as well as its structure and interaction with its receptor. A major part of this article focuses on the regulation of the Epo gene and the discovery of HIF, a transcription factor that plays a cardinal role in molecular adaptation to hypoxia. In the concluding section, a synopsis of Epo's role in disorders of red blood cell production will be followed by an assessment of the remarkable impact of Epo therapy in the treatment of anemias, as well as concerns that provide a strong impetus for the development of even safer and more effective treatment.
Figures

References
-
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, et al. 2002. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet 32: 614–621 - PubMed
-
- Bachmann S, Le Hir M, Eckardt K 1993. Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41: 335–341 - PubMed
-
- Bahlmann FH, DeGroot K, Duckert T, Niemczyk E, Bahlmann E, Boehm SM, Haller H, Fliser D 2003. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int 64: 1648–1652 - PubMed
-
- Bahlmann FH, Song R, Boehm SM, Mengel M, von Wasielewski R, Lindschau C, Kirsch T, de Groot K, Laudeley R, Niemczyk E, et al. 2004. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin α persistently activates endothelial Akt and attenuates progressive organ failure. Circulation 110: 1006–1012 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
