Human genetic disorders caused by mutations in genes encoding biosynthetic enzymes for sulfated glycosaminoglycans
- PMID: 23457301
- PMCID: PMC3630846
- DOI: 10.1074/jbc.R112.437038
Human genetic disorders caused by mutations in genes encoding biosynthetic enzymes for sulfated glycosaminoglycans
Abstract
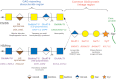
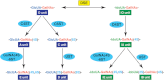
A number of genetic disorders are caused by mutations in the genes encoding glycosyltransferases and sulfotransferases, enzymes responsible for the synthesis of sulfated glycosaminoglycan (GAG) side chains of proteoglycans, including chondroitin sulfate, dermatan sulfate, and heparan sulfate. The phenotypes of these genetic disorders reflect disturbances in crucial biological functions of GAGs in human. Recent studies have revealed that mutations in genes encoding chondroitin sulfate and dermatan sulfate biosynthetic enzymes cause various disorders of connective tissues. This minireview focuses on growing glycobiological studies of recently described genetic diseases caused by disturbances in biosynthetic enzymes for sulfated GAGs.
Figures
References
-
- Iozzo R. V. (1998) Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 67, 609–652 - PubMed
-
- Sugahara K., Kitagawa H. (2000) Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 10, 518–527 - PubMed
-
- Mizumoto S., Sugahara K. (2012) Bone and skin disorders caused by a disturbance in the biosynthesis of chondroitin sulfate and dermatan sulfate. in Extracellular Matrix: Pathobiology and Signaling (Karamanos N., ed) pp. 97–118, Walter De Gruyter, Berlin
-
- Sugahara K., Mikami T., Uyama T., Mizuguchi S., Nomura K., Kitagawa H. (2003) Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 13, 612–620 - PubMed
-
- Sugahara K., Mikami T. (2007) Chondroitin/dermatan sulfate in the central nervous system. Curr. Opin. Struct. Biol. 17, 536–545 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

