Platelet-derived growth factor-BB activates calcium/calmodulin-dependent and -independent mechanisms that mediate Akt phosphorylation in the neurofibromin-deficient human Schwann cell line ST88-14
- PMID: 23457304
- PMCID: PMC3630876
- DOI: 10.1074/jbc.M112.442244
Platelet-derived growth factor-BB activates calcium/calmodulin-dependent and -independent mechanisms that mediate Akt phosphorylation in the neurofibromin-deficient human Schwann cell line ST88-14
Abstract
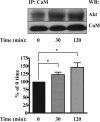
Neurofibromatosis type 1-derived Schwann cells isolated from malignant peripheral nerve sheath tumors (MPNSTs) overexpress PDGF receptor-β and generate an aberrant intracellular calcium increase in response to PDGF-BB. Using the human MPNST Schwann cell line ST88-14, we demonstrate that, in addition to a transient phosphorylation of Akt, PDGF-BB stimulation produces an atypical sustained phosphorylation of Akt that is dependent on calcium and calmodulin (CaM). The sustained Akt phosphorylation did not occur in PDGF-BB-stimulated normal human Schwann cells or ST88-14 cells stimulated with stem cell factor, whose receptor is also overexpressed in ST88-14 cells. The sustained Akt phosphorylation induced by PDGF-BB was inhibited by pretreatment of the cells with either the intracellular calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl) ester (BAPTA-AM) or the CaM antagonist W7, whereas the transient portion was not inhibited. Akt also co-immunoprecipitated with CaM in a PDGF-BB-dependent manner, suggesting that direct interaction between Akt and CaM is involved in the sustained phosphorylation of Akt. Furthermore, we provide evidence that anti-apoptotic effects of PDGF-BB on serum-deprived ST88-14 cells can be inhibited by W7, implicating the PDGF-BB-induced activation of calcium/CaM in promoting cell survival, presumably through sustained Akt activation. We conclude that the activation of the calcium/CaM/Akt pathway resulting from stimulation of overexpressed PDGF receptor-β may contribute to the survival and tumorigenicity of MPNST cells.
Figures




Similar articles
-
Schwann cell lines derived from malignant peripheral nerve sheath tumors respond abnormally to platelet-derived growth factor-BB.J Neurosci Res. 2005 Feb 1;79(3):318-28. doi: 10.1002/jnr.20334. J Neurosci Res. 2005. PMID: 15602756
-
The role of platelet-derived growth factor-BB-induced increase in cytosolic free Ca2+ in activation of mitogen-activated protein kinase and DNA synthesis in vascular smooth muscle cells.J Hypertens. 1997 Dec;15(12 Pt 2):1671-5. doi: 10.1097/00004872-199715120-00071. J Hypertens. 1997. PMID: 9488221
-
Src tyrosine kinase mediates platelet-derived growth factor BB-induced and redox-dependent migration in metanephric mesenchymal cells.Am J Physiol Renal Physiol. 2014 Jan 1;306(1):F85-97. doi: 10.1152/ajprenal.00371.2013. Epub 2013 Nov 6. Am J Physiol Renal Physiol. 2014. PMID: 24197068 Free PMC article.
-
The Fer tyrosine kinase is important for platelet-derived growth factor-BB-induced signal transducer and activator of transcription 3 (STAT3) protein phosphorylation, colony formation in soft agar, and tumor growth in vivo.J Biol Chem. 2013 May 31;288(22):15736-44. doi: 10.1074/jbc.M113.476424. Epub 2013 Apr 15. J Biol Chem. 2013. PMID: 23589302 Free PMC article.
-
A Promising Candidate in Tendon Healing Events-PDGF-BB.Biomolecules. 2022 Oct 20;12(10):1518. doi: 10.3390/biom12101518. Biomolecules. 2022. PMID: 36291727 Free PMC article. Review.
Cited by
-
Dynamic Visualization of mTORC1 Activity in Living Cells.Cell Rep. 2015 Mar 17;10(10):1767-1777. doi: 10.1016/j.celrep.2015.02.031. Epub 2015 Mar 12. Cell Rep. 2015. PMID: 25772363 Free PMC article.
-
A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor.Nat Rev Cancer. 2015 May;15(5):290-301. doi: 10.1038/nrc3911. Epub 2015 Apr 16. Nat Rev Cancer. 2015. PMID: 25877329 Free PMC article. Review.
-
Fast and robust next-generation sequencing technique using ion torrent personal genome machine for the screening of neurofibromatosis type 1 (NF1) gene.J Mol Neurosci. 2014 Jun;53(2):204-10. doi: 10.1007/s12031-014-0286-7. Epub 2014 Mar 28. J Mol Neurosci. 2014. PMID: 24676943
References
-
- Carroll S. L., Stonecypher M. S. (2005) Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors. II. The role of dysregulated growth factor signaling. J. Neuropathol. Exp. Neurol. 64, 1–9 - PubMed
-
- Thomas S. L., De Vries G. H. (2009) Neurofibromatosis type I: from genetic mutation to tumor formation. in Handbook of Neurochemistry and Molecular Neurobiology (Lajtha A., ed) 3rd Ed., pp. 107–130, Springer, New York
-
- Badache A., Muja N., De Vries G. H. (1998) Expression of Kit in neurofibromin-deficient human Schwann cells: role in Schwann cell hyperplasia associated with type 1 neurofibromatosis. Oncogene 17, 795–800 - PubMed
-
- Badache A., De Vries G. H. (1998) Neurofibrosarcoma-derived Schwann cells overexpress platelet-derived growth factor (PDGF) receptors and are induced to proliferate by PDGF BB. J. Cell. Physiol. 177, 334–342 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

