Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease
- PMID: 23457378
- PMCID: PMC3676865
- DOI: 10.3899/jrheum.121043
Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease
Abstract
Objective: Small series suggest mycophenolate mofetil (MMF) is well tolerated and may be an effective therapy for connective tissue disease-associated interstitial lung disease (CTD-ILD). We examined the tolerability and longitudinal changes in pulmonary physiology in a large and diverse cohort of patients with CTD-ILD treated with MMF.
Methods: We identified consecutive patients evaluated at our center between January 2008 and January 2011 and prescribed MMF for CTD-ILD. We assessed safety and tolerability of MMF and used longitudinal data analyses to examine changes in pulmonary physiology over time, before and after initiation of MMF.
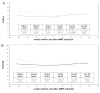
Results: We identified 125 subjects treated with MMF for a median 897 days. MMF was discontinued in 13 subjects. MMF was associated with significant improvements in estimated percentage of predicted forced vital capacity (FVC%) from MMF initiation to 52, 104, and 156 weeks (4.9% ± 1.9%, p = 0.01; 6.1% ± 1.8%, p = 0.0008; and 7.3% ± 2.6%, p = 0.004, respectively); and in estimated percentage predicted diffusing capacity (DLCO%) from MMF initiation to 52 and 104 weeks (6.3% ± 2.8%, p = 0.02; 7.1% ± 2.8%, p = 0.01). In the subgroup without usual interstitial pneumonia (UIP)-pattern injury, MMF significantly improved FVC% and DLCO%, and in the subgroup with UIP-pattern injury, MMF was associated with stability in FVC% and DLCO%.
Conclusion: In a large diverse cohort of CTD-ILD, MMF was well tolerated and had a low rate of discontinuation. Treatment with MMF was associated with either stable or improved pulmonary physiology over a median 2.5 years of followup. MMF appears to be a promising therapy for the spectrum of CTD-ILD.
Keywords: CONNECTIVE TISSUE DISEASE; INTERSTITIAL LUNG DISEASE; MYCOPHENOLATE MOFETIL.
Figures



Comment in
-
Can large simple trials help us understand when and how to use generic drugs for uncommon diseases?J Rheumatol. 2013 May;40(5):539-41. doi: 10.3899/jrheum.130314. J Rheumatol. 2013. PMID: 23637373 No abstract available.
References
-
- Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. - PubMed
-
- Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. - PubMed
-
- Swigris JJ, Olson AL, Fischer A, Lynch DA, Cosgrove GP, Frankel SK, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissuedisease-related interstitial lung disease. Chest. 2006;130:30–6. - PubMed
-
- Gerbino AJ, Goss CH, Molitor JA. Effect of mycophenolate mofetil on pulmonary function in scleroderma-associated interstitial lung disease. Chest. 2008;133:455–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
