Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia
- PMID: 23457487
- PMCID: PMC3572185
- DOI: 10.1371/journal.pone.0055934
Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia
Abstract
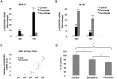
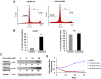
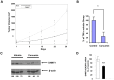
Bioactive components from dietary supplements such as curcumin may represent attractive agents for cancer prevention or treatment. DNA methylation plays a critical role in acute myeloid leukemia (AML) development, and presents an excellent target for treatment of this disease. However, it remains largely unknown how curcumin, a component of the popular Indian spice turmeric, plays a role in DNA hypomethylation to reactivate silenced tumor suppressor genes and to present a potential treatment option for AML. Here we show that curcumin down-regulates DNMT1 expression in AML cell lines, both in vitro and in vivo, and in primary AML cells ex vivo. Mechanistically, curcumin reduced the expression of positive regulators of DNMT1, p65 and Sp1, which correlated with a reduction in binding of these transcription factors to the DNMT1 promoter in AML cell lines. This curcumin-mediated down-regulation of DNMT1 expression was concomitant with p15(INK4B) tumor suppressor gene reactivation, hypomethylation of the p15(INK4B) promoter, G1 cell cycle arrest, and induction of tumor cell apoptosis in vitro. In mice implanted with the human AML MV4-11 cell line, administration of curcumin resulted in remarkable suppression of AML tumor growth. Collectively, our data indicate that curcumin shows promise as a potential treatment for AML, and our findings provide a basis for future studies to test the clinical efficacy of curcumin - whether used as a single agent or as an adjuvant - for AML treatment.
Conflict of interest statement
Figures


References
-
- Baylin SB (2005) DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol 2 Suppl 1S4–11. - PubMed
-
- Jones PA, Baylin SB (2002) The fundamental role of epigenetic events in cancer. Nat Rev Genet 3: 415–428. - PubMed
-
- Issa JP (2007) DNA methylation as a therapeutic target in cancer. Clin Cancer Res 13: 1634–1637. - PubMed
-
- Yoo CB, Jones PA (2006) Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov 5: 37–50. - PubMed
-
- Blum W, Marcucci G (2005) Targeting epigenetic changes in acute myeloid leukemia. Clin Adv Hematol Oncol 3: 855–865, 882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

