Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathways
- PMID: 23457620
- PMCID: PMC3572951
- DOI: 10.1371/journal.pone.0056819
Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathways
Abstract
Purpose: In diseases such as proliferative vitreoretinopathy (PVR), proliferative diabetic retinopathy, and age-related macular degeneration, retinal pigment epithelial (RPE) cells proliferate and migrate. Moreover, platelet-derived growth factor (PDGF) has been shown to enhance proliferation and migration of RPE cells in PVR. Even resveratrol can suppress the migration and adhesion of many cell types, its effects on RPE cell migration and adhesion remain unknown. In this study, we investigated the inhibitory effects of resveratrol on RPE cell migration induced by PDGF-BB, an isoform of PDGF, and adhesion to fibronectin, a major ECM component of PVR tissue.
Methods: The migration of RPE cells was assessed by an electric cell-substrate impedance sensing migration assay and a Transwell migration assay. A cell viability assay was used to determine the viability of resveratrol treated-cells. The cell adhesion to fibronectin was examined by an adhesion assay. The interactions of resveratrol with PDGF-BB were analyzed by a dot binding assay. The PDGF-BB-induced signaling pathways were determined by western blotting and scratch wound healing assay.
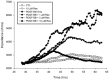
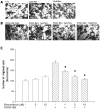
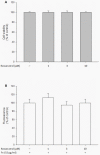
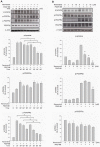
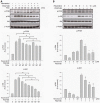
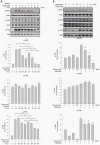
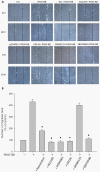
Results: Resveratrol inhibited PDGF-BB-induced RPE cell migration in a dose-dependent manner, but showed no effects on ARPE19 cell adhesion to fibronectin. The cell viability assay showed no cytotoxicity of resveratrol on RPE cells and the dot binding assay revealed no direct interactions of resveratrol with PDGF-BB. Inhibitory effects of resveratrol on PDGF-BB-induced platelet-derived growth factor receptor β (PDGFRβ) and tyrosine phosphorylation and the underlying pathways of PI3K/Akt, ERK and p38 activation were found; however, resveratrol and PDGF-BB showed no effects on PDGFRα and JNK activation. Scratch wound healing assay demonstrated resveratrol and the specific inhibitors of PDGFR, PI3K, MEK or p38 suppressed PDGF-BB-induced cell migration.
Conclusions: These results indicate that resveratrol is an effective inhibitor of PDGF-BB-induced RPE cell migration via PDGFRβ, PI3K/Akt and MAPK pathways, but has no effects on the RPE cell adhesion to fibronectin.
Conflict of interest statement
Figures

References
-
- Charteris DG, Sethi CS, Lewis GP, Fisher SK (2002) Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye (Lond) 16: 369–374. - PubMed
-
- Cardillo JA, Stout JT, LaBree L, Azen SP, Omphroy L, et al. (1997) Post-traumatic proliferative vitreoretinopathy. The epidemiologic profile, onset, risk factors, and visual outcome. Ophthalmology 104: 1166–1173. - PubMed
-
- Campochiaro PA (1997) Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol 115: 237–241. - PubMed
-
- de Silva DJ, Kwan A, Bunce C, Bainbridge J (2008) Predicting visual outcome following retinectomy for retinal detachment. Br J Ophthalmol 92: 954–958. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

