Regulation of the membrane insertion and conductance activity of the metamorphic chloride intracellular channel protein CLIC1 by cholesterol
- PMID: 23457643
- PMCID: PMC3572944
- DOI: 10.1371/journal.pone.0056948
Regulation of the membrane insertion and conductance activity of the metamorphic chloride intracellular channel protein CLIC1 by cholesterol
Abstract
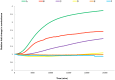
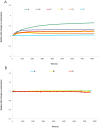
The Chloride Intracellular ion channel protein CLIC1 has the ability to spontaneously insert into lipid membranes from a soluble, globular state. The precise mechanism of how this occurs and what regulates this insertion is still largely unknown, although factors such as pH and redox environment are known contributors. In the current study, we demonstrate that the presence and concentration of cholesterol in the membrane regulates the spontaneous insertion of CLIC1 into the membrane as well as its ion channel activity. The study employed pressure versus area change measurements of Langmuir lipid monolayer films; and impedance spectroscopy measurements using tethered bilayer membranes to monitor membrane conductance during and following the addition of CLIC1 protein. The observed cholesterol dependent behaviour of CLIC1 is highly reminiscent of the cholesterol-dependent-cytolysin family of bacterial pore-forming proteins, suggesting common regulatory mechanisms for spontaneous protein insertion into the membrane bilayer.
Conflict of interest statement
Figures



References
-
- Valenzuela S, Martin DK, Por SB, Robbins JM, Bootcov MR, et al. (1996) NCC27 - A novel nuclear chloride ion channel associated with macrophage activation. Journal Of Leukocyte Biology 106–106. - PubMed
-
- Berryman M, Bruno J, Price J, Edwards JC (2004) CLIC-5A functions as a chloride channel in vitro and associates with the cortical actin cytoskeleton in vitro and in vivo. The Journal of Biological Chemistry 279: 34794–34801. - PubMed
-
- Cromer BA, Gorman MA, Hansen G, Adams JJ, Coggan M, et al. (2007) Structure of the Janus protein human CLIC2. Journal Of Molecular Biology 374: 719–731. - PubMed
-
- Harrop SJ, DeMaere MZ, Fairlie WD, Reztsova T, Valenzuela SM, et al. (2001) Crystal Structure of a Soluble Form of the Intracellular Chloride Ion Channel CLIC1 (NCC27) at 1.4-Å Resolution. Journal Of Biological Chemistry 276: 44993–45000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

