Comparison of sensory neuron growth cone and filopodial responses to structurally diverse aggrecan variants, in vitro
- PMID: 23458191
- PMCID: PMC3778668
- DOI: 10.1016/j.expneurol.2013.02.012
Comparison of sensory neuron growth cone and filopodial responses to structurally diverse aggrecan variants, in vitro
Abstract
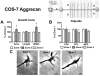
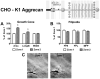
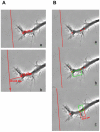
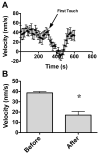
Following spinal cord injury, a regenerating neurite encounters a glial scar enriched in chondroitin sulfate proteoglycans (CSPGs), which presents a major barrier. There are two points at which a neurite makes contact with glial scar CSPGs: initially, filopodia surrounding the growth cone extend and make contact with CSPGs, then the peripheral domain of the entire growth cone makes CSPG contact. Aggrecan is a CSPG commonly used to model the effect CSPGs have on elongating or regenerating neurites. In this study, we investigated filopodia and growth cone responses to contact with structurally diverse aggrecan variants using the common stripe assay. Using time-lapse imaging with 15-s intervals, we measured growth cone area, growth cone width, growth cone length, filopodia number, total filopodia length, and the length of the longest filopodia following contact with aggrecan. Responses were measured after both filopodia and growth cone contact with five different preparations of aggrecan: two forms of aggrecan derived from bovine articular cartilage (purified and prepared using different techniques), recombinant aggrecan lacking chondroitin sulfate side chains (produced in CHO-745 cells) and two additional recombinant aggrecan preparations with varying lengths of chondroitin sulfate side chains (produced in CHO-K1 and COS-7 cells). Responses in filopodia and growth cone behavior differed between the structurally diverse aggrecan variants. Mutant CHO-745 aggrecan (lacking chondroitin sulfate chains) permitted extensive growth across the PG stripe. Filopodia contact with the CHO-745 aggrecan caused a significant increase in growth cone width and filopodia length (112.7% ± 4.9 and 150.9% ± 7.2 respectively, p<0.05), and subsequently upon growth cone contact, growth cone width remained elevated along with a reduction in filopodia number (121.9% ± 4.2; 72.39% ± 6.4, p<0.05). COS-7 derived aggrecan inhibited neurite outgrowth following growth cone contact. Filopodia contact produced an increase in growth cone area and width (126.5% ± 8.1; 150.3% ± 13.31, p<0.001), and while these parameters returned to baseline upon growth cone contact, a reduction in filopodia number and length was observed (73.94% ± 5.8, 75.3% ± 6.2, p<0.05). CHO-K1 derived aggrecan inhibited neurite outgrowth following filopodia contact, and caused an increase in growth cone area and length (157.6% ± 6.2; 117.0% ± 2.8, p<0.001). Interestingly, the two bovine articular cartilage aggrecan preparations differed in their effects on neurite outgrowth. The proprietary aggrecan (BA I, Sigma-Aldrich) inhibited neurites at the point of growth cone contact, while our chemically purified aggrecan (BA II) inhibited neurite outgrowth at the point of filopodia contact. BA I caused a reduction in growth cone width following filopodia contact (91.7% ± 2.5, p<0.05). Upon growth cone contact, there was a further reduction in growth cone width and area (66.4% ± 2.2; 75.6% ± 2.9; p<0.05), as well as reductions in filopodia number, total length, and max length (75.9% ± 5.7, p<0.05; 68.8% ± 6.0; 69.6% ± 3.5, p<0.001). Upon filopodia contact, BA II caused a significant increase in growth cone area, and reductions in filopodia number and total filopodia length (115.9% ± 5.4, p<0.05; 72.5% ± 2.7; 77.7% ± 3.2, p<0.001). In addition, filopodia contact with BA I caused a significant reduction in growth cone velocity (38.6 nm/s ± 1.3 before contact, 17.1 nm/s ± 3.6 after contact). These data showed that neuron morphology and behavior are differentially dependent upon aggrecan structure. Furthermore, the behavioral changes associated with the approaching growth cone may be predictive of inhibition or growth.
Keywords: BA; Bovine (steer) articular cartilage aggrecan; CHO-K1; COS7; CSPG; Cell culture; Chinese hamster ovary K1 (cell line); Chondroitin sulfate proteoglycan; Dorsal root ganglia neurons; Extracellular matrix (ECM); Neuronal growth cones; Regeneration; Simian kidney cell line; Velocity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Growth cone behavior in the presence of soluble chondroitin sulfate proteoglycan (CSPG), compared to behavior on CSPG bound to laminin or fibronectin.Int J Dev Neurosci. 1996 Jun;14(3):331-49. doi: 10.1016/0736-5748(96)00017-2. Int J Dev Neurosci. 1996. PMID: 8842808
-
Nervous system-derived chondroitin sulfate proteoglycans regulate growth cone morphology and inhibit neurite outgrowth: a light, epifluorescence, and electron microscopy study.Microsc Res Tech. 2001 Sep 1;54(5):273-86. doi: 10.1002/jemt.1140. Microsc Res Tech. 2001. PMID: 11514984
-
Membrane-bound CSPG mediates growth cone outgrowth and substrate specificity by Schwann cell contact with the DRG neuron cell body and not via growth cone contact.Exp Neurol. 2006 Jul;200(1):19-25. doi: 10.1016/j.expneurol.2006.02.001. Epub 2006 Mar 10. Exp Neurol. 2006. PMID: 16530184
-
Targeting Chondroitin Sulfate Proteoglycans: An Emerging Therapeutic Strategy to Treat CNS Injury.ACS Chem Neurosci. 2020 Feb 5;11(3):231-232. doi: 10.1021/acschemneuro.0c00004. Epub 2020 Jan 15. ACS Chem Neurosci. 2020. PMID: 31939650 Review.
-
Aggrecan in Cardiovascular Development and Disease.J Histochem Cytochem. 2020 Nov;68(11):777-795. doi: 10.1369/0022155420952902. Epub 2020 Sep 1. J Histochem Cytochem. 2020. PMID: 32870742 Free PMC article. Review.
Cited by
-
Intact glycosaminoglycans from intervertebral disc-derived notochordal cell-conditioned media inhibit neurite growth while maintaining neuronal cell viability.Spine J. 2015 May 1;15(5):1060-9. doi: 10.1016/j.spinee.2015.02.003. Epub 2015 Feb 7. Spine J. 2015. PMID: 25661435 Free PMC article.
-
Contributions of Chondroitin Sulfate, Keratan Sulfate and N-linked Oligosaccharides to Inhibition of Neurite Outgrowth by Aggrecan.Biology (Basel). 2020 Feb 12;9(2):29. doi: 10.3390/biology9020029. Biology (Basel). 2020. PMID: 32059349 Free PMC article.
-
An in vitro assay to study induction of the regenerative state in sensory neurons.Exp Neurol. 2015 Jan;263:350-63. doi: 10.1016/j.expneurol.2014.10.012. Epub 2014 Nov 4. Exp Neurol. 2015. PMID: 25447942 Free PMC article.
-
Demystifying the extracellular matrix and its proteolytic remodeling in the brain: structural and functional insights.Cell Mol Life Sci. 2019 Aug;76(16):3229-3248. doi: 10.1007/s00018-019-03182-6. Epub 2019 Jun 13. Cell Mol Life Sci. 2019. PMID: 31197404 Free PMC article. Review.
-
Proteoglycans: road signs for neurite outgrowth.Neural Regen Res. 2014 Feb 15;9(4):343-55. doi: 10.4103/1673-5374.128235. Neural Regen Res. 2014. PMID: 25206822 Free PMC article.
References
-
- Asher RA, Fidler PS, Rogers JH, Fawcett JW. TGF-ß stimulates neurocan synthesis in cultured rat astrocytes. Soc for Neurosci Abst. 1998;24:56.
-
- Barbosa I, Garcia S, Barbier-Chassefiere V, Caruelle JP, Martelly I, Papy-Garcia D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13:647–653. - PubMed
-
- Beller JA, Gurkoff GG, Berman RF, Lyeth BG. Pharmacological enhancement of glutamate transport reduces excitotoxicity in vitro. Restor Neurol Neurosci. 2011;29:331–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

