Phosphoryl transfer by protein kinase A is captured in a crystal lattice
- PMID: 23458248
- PMCID: PMC3663052
- DOI: 10.1021/ja312237q
Phosphoryl transfer by protein kinase A is captured in a crystal lattice
Abstract
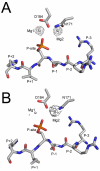
The catalytic (C) subunit of cAMP-dependent protein kinase (PKA) is a serine/threonine kinase responsible for most of the effects of cAMP signaling, and PKA serves as a prototype for the entire kinase family. Despite multiple studies of PKA, the steps involved in phosphoryl transfer, the roles of the catalytically essential magnesium ions, and the processes that govern the rate-limiting step of ADP release are unresolved. Here we identified conditions that yielded slow phosphoryl transfer of the γ-phosphate from the generally nonhydrolyzable analog of ATP, adenosine-5'-(β,γ-imido)triphosphate (AMP-PNP), onto a substrate peptide within protein crystals. By trapping both products in the crystal lattice, we now have a complete resolution profile of all the catalytic steps. One crystal structure refined to 1.55 Å resolution shows two states of the protein with 55% displaying intact AMP-PNP and an unphosphorylated substrate and 45% displaying transfer of the γ-phosphate of AMP-PNP onto the substrate peptide yielding AMP-PN and a phosphorylated substrate. Another structure refined to 2.15 Å resolution displays complete phosphoryl transfer to the substrate. These structures, in addition to trapping both products in the crystal lattice, implicate one magnesium ion, previously termed Mg2, as the more stably bound ion. Following phosphoryl transfer, Mg2 recruits a water molecule to retain an octahedral coordination geometry suggesting the strong binding character of this magnesium ion, and Mg2 remains in the active site following complete phosphoryl transfer while Mg1 is expelled. Loss of Mg1 may thus be an important part of the rate-limiting step of ADP release.
Figures





Similar articles
-
Low- and room-temperature X-ray structures of protein kinase A ternary complexes shed new light on its activity.Acta Crystallogr D Biol Crystallogr. 2012 Jul;68(Pt 7):854-60. doi: 10.1107/S0907444912014886. Epub 2012 Jun 15. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 22751671 Free PMC article.
-
Two structures of the catalytic domain of phosphorylase kinase: an active protein kinase complexed with substrate analogue and product.Structure. 1995 May 15;3(5):467-82. doi: 10.1016/s0969-2126(01)00180-0. Structure. 1995. PMID: 7663944
-
Insights into the phosphoryl transfer catalyzed by cAMP-dependent protein kinase: an X-ray crystallographic study of complexes with various metals and peptide substrate SP20.Biochemistry. 2013 May 28;52(21):3721-7. doi: 10.1021/bi400066a. Epub 2013 May 14. Biochemistry. 2013. PMID: 23672593 Free PMC article.
-
Analysis of the phosphoryl transfer mechanism of c-AMP dependent protein kinase (PKA) by penta-coodinate phosphoric transition state theory.Curr Protein Pept Sci. 2005 Oct;6(5):437-42. doi: 10.2174/138920305774329296. Curr Protein Pept Sci. 2005. PMID: 16248795 Review.
-
PKA: a portrait of protein kinase dynamics.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):259-69. doi: 10.1016/j.bbapap.2003.11.029. Biochim Biophys Acta. 2004. PMID: 15023366 Review.
Cited by
-
Functional Role of Histidine in the Conserved His-x-Asp Motif in the Catalytic Core of Protein Kinases.Sci Rep. 2015 May 11;5:10115. doi: 10.1038/srep10115. Sci Rep. 2015. PMID: 25960268 Free PMC article.
-
Edmond Fischer's kinase legacy: History of the protein kinase inhibitor and protein kinase A.IUBMB Life. 2023 Apr;75(4):311-323. doi: 10.1002/iub.2714. Epub 2023 Feb 28. IUBMB Life. 2023. PMID: 36855225 Free PMC article.
-
Divalent Metal Ions Mg²⁺ and Ca²⁺ Have Distinct Effects on Protein Kinase A Activity and Regulation.ACS Chem Biol. 2015 Oct 16;10(10):2303-15. doi: 10.1021/acschembio.5b00271. Epub 2015 Aug 5. ACS Chem Biol. 2015. PMID: 26200257 Free PMC article.
-
Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy.Open Life Sci. 2024 Jul 24;19(1):20220923. doi: 10.1515/biol-2022-0923. eCollection 2024. Open Life Sci. 2024. PMID: 39071492 Free PMC article.
-
Structural and biochemical basis of Arabidopsis FERONIA receptor kinase-mediated early signaling initiation.Plant Commun. 2023 Jul 10;4(4):100559. doi: 10.1016/j.xplc.2023.100559. Epub 2023 Feb 11. Plant Commun. 2023. PMID: 36774537 Free PMC article.
References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298:1912. - PubMed
-
- Taylor SS, Kornev AP. In: PKA: Prototype for Dynamic Signaling in Time and Space. Quantitative Biology: From Molecular to Cellular Systems. Wall ME, editor. CRC Press; Boca Raton, London, New York: 2012. pp. 267–298.
-
- Tasken K, Skalhegg BS, Tasken KA, Solberg R, Knutsen HK, Levy FO, Sandberg M, Orstavik S, Larsen T, Johansen AK, Vang T, Schrader HP, Reinton NT, Torgersen KM, Hansson V, Jahnsen T. Adv Second Messenger Phosphoprotein Res. 1997;31:191. - PubMed
-
- Johnson DA, Akamine P, Radzio-Andzelm E, Madhusudan M, Taylor SS. Chem Rev. 2001;101:2243. - PubMed
-
- Kim C, Cheng CY, Saldanha SA, Taylor SS. Cell. 2007;130:1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

