Concepts and practices used to develop functional PLGA-based nanoparticulate systems
- PMID: 23459088
- PMCID: PMC3582541
- DOI: 10.2147/IJN.S40579
Concepts and practices used to develop functional PLGA-based nanoparticulate systems
Abstract
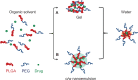
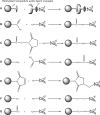
The functionality of bare polylactide-co-glycolide (PLGA) nanoparticles is limited to drug depot or drug solubilization in their hard cores. They have inherent weaknesses as a drug-delivery system. For instance, when administered intravenously, the nanoparticles undergo rapid clearance from systemic circulation before reaching the site of action. Furthermore, plain PLGA nanoparticles cannot distinguish between different cell types. Recent research shows that surface functionalization of nanoparticles and development of new nanoparticulate dosage forms help overcome these delivery challenges and improve in vivo performance. Immense research efforts have propelled the development of diverse functional PLGA-based nanoparticulate delivery systems. Representative examples include PEGylated micelles/nanoparticles (PEG, polyethylene glycol), polyplexes, polymersomes, core-shell-type lipid-PLGA hybrids, cell-PLGA hybrids, receptor-specific ligand-PLGA conjugates, and theranostics. Each PLGA-based nanoparticulate dosage form has specific features that distinguish it from other nanoparticulate systems. This review focuses on fundamental concepts and practices that are used in the development of various functional nanoparticulate dosage forms. We describe how the attributes of these functional nanoparticulate forms might contribute to achievement of desired therapeutic effects that are not attainable using conventional therapies. Functional PLGA-based nanoparticulate systems are expected to deliver chemotherapeutic, diagnostic, and imaging agents in a highly selective and effective manner.
Keywords: functionality; nanoparticles; nanoparticulate dosage forms; polylactide-co-glycolide.
Figures
References
-
- Panyama J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–347. - PubMed
-
- Danhier F, Ansorena E, Silva JM, Coco R, Breton AL, Préat V. PLGA-based nanoparticles: An overview of biomedical applications. J Control Release. 2012;161(2):505–522. - PubMed
-
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. - PubMed
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

