Transformation of context-dependent sensory dynamics into motor behavior
- PMID: 23459114
- PMCID: PMC3572992
- DOI: 10.1371/journal.pcbi.1002908
Transformation of context-dependent sensory dynamics into motor behavior
Abstract
The intrinsic dynamics of sensory networks play an important role in the sensory-motor transformation. In this paper we use conductance based models and electrophysiological recordings to address the study of the dual role of a sensory network to organize two behavioral context-dependent motor programs in the mollusk Clione limacina. We show that: (i) a winner take-all dynamics in the gravimetric sensory network model drives the typical repetitive rhythm in the wing central pattern generator (CPG) during routine swimming; (ii) the winnerless competition dynamics of the same sensory network organizes the irregular pattern observed in the wing CPG during hunting behavior. Our model also shows that although the timing of the activity is irregular, the sequence of the switching among the sensory cells is preserved whenever the same set of neurons are activated in a given time window. These activation phase locks in the sensory signals are transformed into specific events in the motor activity. The activation phase locks can play an important role in motor coordination driven by the intrinsic dynamics of a multifunctional sensory organ.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
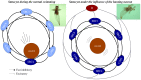
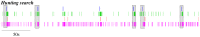

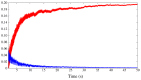
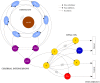


 . Different colors are used to indicate each neuron. The episodes labeled as type A correspond to long activations of neurons 1 and 4 (red and magenta, respectively). The episodes labeled as type B correspond to sequential activations of similar length in all neurons, while type C episodes correspond to long activations of SRCs 2, 3 and 5 (green, blue and cyan). The bottom panels display the first three principal components for the activity of the six receptor cells (left plot) and the four motoneurons in the wing CPG (right plot). The PCA shows that a similar sequential activity in the sensory network during hunting (different hunting episodes of the same type) evokes similar rhythmic activity in the motor system.
. Different colors are used to indicate each neuron. The episodes labeled as type A correspond to long activations of neurons 1 and 4 (red and magenta, respectively). The episodes labeled as type B correspond to sequential activations of similar length in all neurons, while type C episodes correspond to long activations of SRCs 2, 3 and 5 (green, blue and cyan). The bottom panels display the first three principal components for the activity of the six receptor cells (left plot) and the four motoneurons in the wing CPG (right plot). The PCA shows that a similar sequential activity in the sensory network during hunting (different hunting episodes of the same type) evokes similar rhythmic activity in the motor system.
Similar articles
-
The role of sensory network dynamics in generating a motor program.J Neurosci. 2005 Oct 19;25(42):9807-15. doi: 10.1523/JNEUROSCI.2249-05.2005. J Neurosci. 2005. PMID: 16237184 Free PMC article.
-
Dual sensory-motor function for a molluskan statocyst network.J Neurophysiol. 2004 Jan;91(1):336-45. doi: 10.1152/jn.00753.2003. Epub 2003 Sep 24. J Neurophysiol. 2004. PMID: 14507988
-
Pharmacologically induced elements of the hunting and feeding behavior in the pteropod mollusk Clione limacina. II. Effects of physostigmine.J Neurophysiol. 1993 Feb;69(2):522-32. doi: 10.1152/jn.1993.69.2.522. J Neurophysiol. 1993. PMID: 8459283
-
Modulation of swimming speed in the pteropod mollusc, Clione limacina: role of a compartmental serotonergic system.Invert Neurosci. 1996 Dec;2(3):157-65. doi: 10.1007/BF02214171. Invert Neurosci. 1996. PMID: 9372161 Review.
-
Analysis of the central pattern generator for swimming in the mollusk Clione.Ann N Y Acad Sci. 1998 Nov 16;860:51-69. doi: 10.1111/j.1749-6632.1998.tb09038.x. Ann N Y Acad Sci. 1998. PMID: 9928301 Review.
Cited by
-
Transient dynamics and rhythm coordination of inferior olive spatio-temporal patterns.Front Neural Circuits. 2013 Sep 5;7:138. doi: 10.3389/fncir.2013.00138. eCollection 2013. Front Neural Circuits. 2013. PMID: 24046731 Free PMC article.
-
Robust dynamical invariants in sequential neural activity.Sci Rep. 2019 Jun 21;9(1):9048. doi: 10.1038/s41598-019-44953-2. Sci Rep. 2019. PMID: 31227793 Free PMC article.
-
Detection of Activation Sequences in Spiking-Bursting Neurons by means of the Recognition of Intraburst Neural Signatures.Sci Rep. 2018 Nov 13;8(1):16726. doi: 10.1038/s41598-018-34757-1. Sci Rep. 2018. PMID: 30425274 Free PMC article.
-
Central nervous system transcriptome of Biomphalaria alexandrina, an intermediate host for schistosomiasis.BMC Res Notes. 2017 Dec 11;10(1):729. doi: 10.1186/s13104-017-3018-6. BMC Res Notes. 2017. PMID: 29228974 Free PMC article.
-
Asymmetry Factors Shaping Regular and Irregular Bursting Rhythms in Central Pattern Generators.Front Comput Neurosci. 2017 Feb 16;11:9. doi: 10.3389/fncom.2017.00009. eCollection 2017. Front Comput Neurosci. 2017. PMID: 28261081 Free PMC article.
References
-
- Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, et al. (2001) Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci 24: 263–297. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

