New tacrine analogs as acetylcholinesterase inhibitors - theoretical study with chemometric analysis
- PMID: 23459299
- PMCID: PMC6270554
- DOI: 10.3390/molecules18032878
New tacrine analogs as acetylcholinesterase inhibitors - theoretical study with chemometric analysis
Abstract
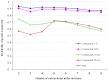
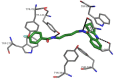
Computer simulations constitute the basis of the design and discovery of new drugs. This approach is not only significant with regards to finding new structures, but also for selecting the molecules with the highest probability of being useful in the diagnostic process and treatment of numerous diseases. In our work, we used computational software to analyze 32 new acetylcholinesterase (AChE) inhibitors and formulate ADMET predictions. To understand the influence of the structure of our derivatives on binding mode, we docked all structures to the active site of AChE and assigned some pharmacophoric features. Finally, we undertook a chemometric analysis of all the compounds on the basis of FT-IR, which gave us the possibility of performing a fast categorization of the analyzed compounds and design compounds with similar structures.
Figures







References
-
- Mikiciuk-Olasik E., Szymański P., Żurek E. Diagnostics and Therapy of Alzheimer’s Disease. Ind. J. Exp. Biol. 2007;45:315–325. - PubMed
-
- Bartus R.T., Dean R.L., Beer B., Lippa A.S. The cholinergic hypothesis of generic memory dysfunction. Science. 1982;217:408–417. - PubMed
-
- Kozurkova M., Hamulakova S., Gazova Z., Paulikova H., Kristian P. Neuroactive multifunctional tacrine congeners with cholinesterase, anti-amyloid aggregation and neuroprotective properties. Pharmaceuticals. 2011;4:382–418.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

