Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents
- PMID: 23459471
- PMCID: PMC3584656
- DOI: 10.2147/BTT.S29965
Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents
Abstract
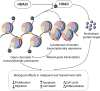
Histone deacetylase (HDAC) inhibitors are an emerging class of therapeutics with potential as anticancer drugs. The rationale for developing HDAC inhibitors (and other chromatin-modifying agents) as anticancer therapies arose from the understanding that in addition to genetic mutations, epigenetic changes such as dysregulation of HDAC enzymes can alter phenotype and gene expression, disturb homeostasis, and contribute to neoplastic growth. The family of HDAC inhibitors is large and diverse. It includes a range of naturally occurring and synthetic compounds that differ in terms of structure, function, and specificity. HDAC inhibitors have multiple cell type-specific effects in vitro and in vivo, such as growth arrest, cell differentiation, and apoptosis in malignant cells. HDAC inhibitors have the potential to be used as monotherapies or in combination with other anticancer therapies. Currently, there are two HDAC inhibitors that have received approval from the US FDA for the treatment of cutaneous T-cell lymphoma: vorinostat (suberoylanilide hydroxamic acid, Zolinza) and depsipeptide (romidepsin, Istodax). More recently, depsipeptide has also gained FDA approval for the treatment of peripheral T-cell lymphoma. Many more clinical trials assessing the effects of various HDAC inhibitors on hematological and solid malignancies are currently being conducted. Despite the proven anticancer effects of particular HDAC inhibitors against certain cancers, many aspects of HDAC enzymes and HDAC inhibitors are still not fully understood. Increasing our understanding of the effects of HDAC inhibitors, their targets and mechanisms of action will be critical for the advancement of these drugs, especially to facilitate the rational design of HDAC inhibitors that are effective as antineoplastic agents. This review will discuss the use of HDAC inhibitors as multitargeted therapies for malignancy. Further, we outline the pharmacology and mechanisms of action of HDAC inhibitors while discussing the safety and efficacy of these compounds in clinical studies to date.
Keywords: chromatin modifications; depsipeptide; entinostat; histone acetylation; histone deacetylase inhibitor; suberoylanilide hydroxamic acid.
Figures
References
-
- Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184(4139):868–871. - PubMed
-
- Kornberg RD. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. - PubMed
-
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. - PubMed
-
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

