Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: implications for host range and adaptation
- PMID: 23459660
- PMCID: PMC3573126
- DOI: 10.1371/journal.ppat.1003151
Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: implications for host range and adaptation
Abstract
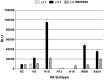
The influenza A virus (IAV) HA protein must be activated by host cells proteases in order to prime the molecule for fusion. Consequently, the availability of activating proteases and the susceptibility of HA to protease activity represents key factors in facilitating virus infection. As such, understanding the intricacies of HA cleavage by various proteases is necessary to derive insights into the emergence of pandemic viruses. To examine these properties, we generated a panel of HAs that are representative of the 16 HA subtypes that circulate in aquatic birds, as well as HAs representative of the subtypes that have infected the human population over the last century. We examined the susceptibility of the panel of HA proteins to trypsin, as well as human airway trypsin-like protease (HAT) and transmembrane protease, serine 2 (TMPRSS2). Additionally, we examined the pH at which these HAs mediated membrane fusion, as this property is related to the stability of the HA molecule and influences the capacity of influenza viruses to remain infectious in natural environments. Our results show that cleavage efficiency can vary significantly for individual HAs, depending on the protease, and that some HA subtypes display stringent selectivity for specific proteases as activators of fusion function. Additionally, we found that the pH of fusion varies by 0.7 pH units among the subtypes, and notably, we observed that the pH of fusion for most HAs from human isolates was lower than that observed from avian isolates of the same subtype. Overall, these data provide the first broad-spectrum analysis of cleavage-activation and membrane fusion characteristics for all of the IAV HA subtypes, and also show that there are substantial differences between the subtypes that may influence transmission among hosts and establishment in new species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Almond JW (1977) A single gene determines the host range of influenza virus. Nature 270: 617–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

